Therapeutic effect of long-acting muscarinic antagonist for treating uncontrolled asthma assessed using impulse oscillometry
- PMID: 39113044
- PMCID: PMC11308707
- DOI: 10.1186/s12931-024-02921-z
Therapeutic effect of long-acting muscarinic antagonist for treating uncontrolled asthma assessed using impulse oscillometry
Abstract
Background: In recent years, the incorporation of LAMAs into asthma therapy has been expected to enhance symptom control. However, a significant number of patients with asthma continue to experience poorly managed symptoms. There have been limited investigations on LAMA-induced airway alterations in asthma treatment employing IOS. In this study, we administered a LAMA to patients with poorly controlled asthma, evaluated clinical responses and respiratory function, and investigated airway changes facilitated by LAMA treatments using the IOS.
Methods: Of a total of 1282 consecutive patients with asthma, 118 exhibited uncontrolled symptoms. Among them, 42 switched their treatment to high-dose fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) (ICS/LABA/LAMA). The patients were then assessed using AHQ-33 or LCQ and ACT. Spirometry parameters (such as FEV1 or MMEF) and IOS parameters (such as R20 or AX) were measured and compared before and after exacerbations and the addition of LAMA.
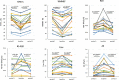
Results: Of the 42 patients, 17 who switched to FF/UMEC/VI caused by dyspnea exhibited decreased pulmonary function between period 1 and baseline, followed by an increase in pulmonary function between baseline and period 2. Significant differences were observed in IOS parameters such as R20, R5-R20, Fres, or AX between period 1 and baseline as well as between baseline and period 2. Among the patients who switched to inhaler due to cough, 25 were classified as responders (n = 17) and nonresponders (n = 8) based on treatment outcomes. Among nonresponders, there were no significant differences in spirometry parameters such as FEV1 or PEF and IOS parameters such as R20 or AX between period 1 and baseline. However, among responders, significant differences were observed in all IOS parameters, though not in most spirometry parameters, between period 1 and baseline. Furthermore, significant differences were noted between baseline and period 2 in terms of FEV1, %MMEF, %PEF, and all IOS parameters.
Conclusion: ICS/LABA/LAMA demonstrates superiority over ICS/LABA in improving symptoms and lung function, which is primarily attributed to the addition of LAMA. Additionally, IOS revealed the effectiveness of LAMA across all airway segments, particularly in the periphery. Hence, LAMA can be effective against various asthma phenotypes characterized by airway inflammation, even in real-world cases.
Keywords: Bronchial asthma; Impulse oscillometry system (IOS); Long-acting muscarinic antagonist (LAMA); Pulmonary function test.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Global initiative for Asthma. Global strategy for asthma management and prevention; 2023. www.ginasthma.org.
-
- Reddel HK, Taylor DR, Bateman ED, et al. An Official American Thoracic Society/European Respiratory Society Statement: Asthma Control and exacerbations. Standardizing endopoints for clinical asthma clinical practice. Am J Respir Crit Care Med. 2009;180:59–99. 10.1164/rccm.200801-060ST - DOI - PubMed
-
- Braido F, Brusselle G, Guastalla D, et al. Determinants and impact of suboptimal asthma control in Europe: the international cross-sectional and longitudinal assessment on asthma control (LIAISON) study. Respir Res. 2016;17:51. 10.1186/s12931-016-0374-z. 10.1186/s12931-016-0374-z - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

