Concomitant targeting of FLT3 and SPHK1 exerts synergistic cytotoxicity in FLT3-ITD+ acute myeloid leukemia by inhibiting β-catenin activity via the PP2A-GSK3β axis
- PMID: 39113090
- PMCID: PMC11304842
- DOI: 10.1186/s12964-024-01774-9
Concomitant targeting of FLT3 and SPHK1 exerts synergistic cytotoxicity in FLT3-ITD+ acute myeloid leukemia by inhibiting β-catenin activity via the PP2A-GSK3β axis
Abstract
Background: Approximately 25-30% of patients with acute myeloid leukemia (AML) have FMS-like receptor tyrosine kinase-3 (FLT3) mutations that contribute to disease progression and poor prognosis. Prolonged exposure to FLT3 tyrosine kinase inhibitors (TKIs) often results in limited clinical responses due to diverse compensatory survival signals. Therefore, there is an urgent need to elucidate the mechanisms underlying FLT3 TKI resistance. Dysregulated sphingolipid metabolism frequently contributes to cancer progression and a poor therapeutic response. However, its relationship with TKI sensitivity in FLT3-mutated AML remains unknown. Thus, we aimed to assess mechanisms of FLT3 TKI resistance in AML.
Methods: We performed lipidomics profiling, RNA-seq, qRT-PCR, and enzyme-linked immunosorbent assays to determine potential drivers of sorafenib resistance. FLT3 signaling was inhibited by sorafenib or quizartinib, and SPHK1 was inhibited by using an antagonist or via knockdown. Cell growth and apoptosis were assessed in FLT3-mutated and wild-type AML cell lines via Cell counting kit-8, PI staining, and Annexin-V/7AAD assays. Western blotting and immunofluorescence assays were employed to explore the underlying molecular mechanisms through rescue experiments using SPHK1 overexpression and exogenous S1P, as well as inhibitors of S1P2, β-catenin, PP2A, and GSK3β. Xenograft murine model, patient samples, and publicly available data were analyzed to corroborate our in vitro results.
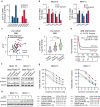
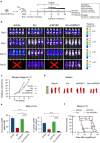
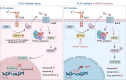
Results: We demonstrate that long-term sorafenib treatment upregulates SPHK1/sphingosine-1-phosphate (S1P) signaling, which in turn positively modulates β-catenin signaling to counteract TKI-mediated suppression of FLT3-mutated AML cells via the S1P2 receptor. Genetic or pharmacological inhibition of SPHK1 potently enhanced the TKI-mediated inhibition of proliferation and apoptosis induction in FLT3-mutated AML cells in vitro. SPHK1 knockdown enhanced sorafenib efficacy and improved survival of AML-xenografted mice. Mechanistically, targeting the SPHK1/S1P/S1P2 signaling synergizes with FLT3 TKIs to inhibit β-catenin activity by activating the protein phosphatase 2 A (PP2A)-glycogen synthase kinase 3β (GSK3β) pathway.
Conclusions: These findings establish the sphingolipid metabolic enzyme SPHK1 as a regulator of TKI sensitivity and suggest that combining SPHK1 inhibition with TKIs could be an effective approach for treating FLT3-mutated AML.
Keywords: Acute myeloid leukemia; FLT3; SPHK1; Sphingolipid; Tyrosine kinase inhibitor; Β-catenin.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

