Therapeutic potential of adipose-derived stem cell extracellular vesicles: from inflammation regulation to tissue repair
- PMID: 39113098
- PMCID: PMC11304935
- DOI: 10.1186/s13287-024-03863-5
Therapeutic potential of adipose-derived stem cell extracellular vesicles: from inflammation regulation to tissue repair
Abstract
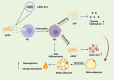
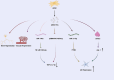
Inflammation is a key pathological feature of many diseases, disrupting normal tissue structure and resulting in irreversible damage. Despite the need for effective inflammation control, current treatments, including stem cell therapies, remain insufficient. Recently, extracellular vesicles secreted by adipose-derived stem cells (ADSC-EVs) have garnered attention for their significant anti-inflammatory properties. As carriers of bioactive substances, these vesicles have demonstrated potent capabilities in modulating inflammation and promoting tissue repair in conditions such as rheumatoid arthritis, osteoarthritis, diabetes, cardiovascular diseases, stroke, and wound healing. Consequently, ADSC-EVs are emerging as promising alternatives to conventional ADSC-based therapies, offering advantages such as reduced risk of immune rejection, enhanced stability, and ease of storage and handling. However, the specific mechanisms by which ADSC-EVs regulate inflammation under pathological conditions are not fully understood. This review discusses the role of ADSC-EVs in inflammation control, their impact on disease prognosis, and their potential to promote tissue repair. Additionally, it provides insights into future clinical research focused on ADSC-EV therapies for inflammatory diseases, which overcome some limitations associated with cell-based therapies.
Keywords: Adipose-derived stem cell; Cell-free therapy; Extracellular vesicles; Inflammation control; Tissue repair.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Igami K, Uchiumi T, Ueda S, Kamioka K, Setoyama D, Gotoh K, Akimoto M, Matsumoto S, Kang D. Characterization and function of medium and large extracellular vesicles from plasma and urine by surface antigens and Annexin V. PeerJ Anal Chem. 2020;2:e4. 10.7717/peerj-achem.4 - DOI
-
- Record M, Carayon K, Poirot M, Silvente-Poirot S. Exosomes as new vesicular lipid transporters involved in cell–cell communication and various pathophysiologies. Biochim et Biophys Acta (BBA)-Molecular Cell Biology Lipids. 2014;1841(1):108–20. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

