The polyunsaturated fatty acid docosahexaenoic affects mitochondrial function in prostate cancer cells
- PMID: 39113152
- PMCID: PMC11308158
- DOI: 10.1186/s40170-024-00348-0
The polyunsaturated fatty acid docosahexaenoic affects mitochondrial function in prostate cancer cells
Abstract
Background: Prostate cancer (PCa) shows a rewired metabolism featuring increased fatty acid uptake and synthesis via de novo lipogenesis, both sharply related to mitochondrial physiology. The docosahexaenoic acid (DHA) is an omega-3 polyunsaturated fatty acid (PUFA) that exerts its antitumoral properties via different mechanisms, but its specific action on mitochondria in PCa is not clear. Therefore, we investigated whether the DHA modulates mitochondrial function in PCa cell lines.
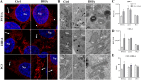
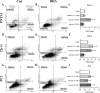
Methods: Here, we evaluated mitochondrial function of non-malignant PNT1A and the castration-resistant (CRPC) prostate 22Rv1 and PC3 cell lines in response to DHA incubation. For this purpose, we used Seahorse extracellular flux assay to assess mitochondria function, [14C]-glucose to evaluate its oxidation as well as its contribution to fatty acid synthesis, 1H-NMR for metabolite profile determination, MitoSOX for superoxide anion production, JC-1 for mitochondrial membrane polarization, mass spectrometry for determination of phosphatidylglycerol levels and composition, staining with MitoTracker dye to assess mitochondrial morphology under super-resolution in addition to Transmission Electron Microscopy, In-Cell ELISA for COX-I and SDH-A protein expression and flow cytometry (Annexin V and 7-AAD) for cell death estimation.
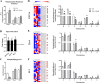
Results: In all cell lines DHA decreased basal respiratory activity, ATP production, and the spare capacity in mitochondria. Also, the omega-3 induced mitochondrial hyperpolarization, ROS overproduction and changes in membrane phosphatidylglycerol composition. In PNT1A, DHA led to mitochondrial fragmentation and it increased glycolysis while in cancer cells it stimulated glucose oxidation, but decreased de novo lipogenesis specifically in 22Rv1, indicating a metabolic shift. In all cell lines, DHA modulated several metabolites related to energy metabolism and it was incorporated in phosphatidylglycerol, a precursor of cardiolipin, increasing the unsaturation index in the mitochondrial membrane. Accordingly, DHA triggered cell death mainly in PNT1A and 22Rv1.
Conclusion: In conclusion, mitochondrial metabolism is significantly affected by the PUFA supplementation to the point that cells are not able to proliferate or survive under DHA-enriched condition. Moreover, combination of DHA supplementation with inhibition of metabolism-related pathways, such as de novo lipogenesis, may be synergistic in castration-resistant prostate cancer.
Keywords: Docosahexaenoic acid; Lipid metabolism; Mitochondria; Omega-3 polyunsaturated fatty acids; Prostate cancer cells.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous

