Different outcomes of endurance and resistance exercise in skeletal muscles of Oculopharyngeal muscular dystrophy
- PMID: 39113268
- PMCID: PMC11446690
- DOI: 10.1002/jcsm.13546
Different outcomes of endurance and resistance exercise in skeletal muscles of Oculopharyngeal muscular dystrophy
Abstract
Background: Exercise is widely considered to have beneficial impact on skeletal muscle aging. In addition, there are also several studies demonstrating a positive effect of exercise on muscular dystrophies. Oculopharyngeal muscular dystrophy (OPMD) is a late-onset autosomal dominant inherited neuromuscular disorder caused by mutations in the PAPBN1 gene. These mutations consist in short (1-8) and meiotically stable GCN trinucleotide repeat expansions in its coding region responsible for the formation of PAPBN1 intranuclear aggregates. This study aims to characterize the effects of two types of chronic exercise, resistance and endurance, on the OPMD skeletal muscle phenotype using a relevant murine model of OPMD.
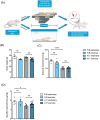
Methods: In this study, we tested two protocols of exercise. In the first, based on endurance exercise, FvB (wild-type) and A17 (OPMD) mice underwent a 6-week-long motorized treadmill protocol consisting in three sessions per week of running 20 cm/s for 20 min. In the second protocol, based on resistance exercise generated by chronic mechanical overload (OVL), surgical removal of gastrocnemius and soleus muscles was performed, inducing hypertrophy of the plantaris muscle. In both types of exercise, muscles of A17 and FvB mice were compared with those of respective sedentary mice. For all the groups, force measurement, muscle histology, and molecular analyses were conducted.
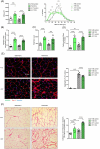
Results: Following the endurance exercise protocol, we did not observe any major changes in the muscle physiological parameters, but an increase in the number of PABPN1 intranuclear aggregates in both tibialis anterior (+24%, **P = 0.0026) and gastrocnemius (+18%, ****P < 0.0001) as well as enhanced collagen deposition (+20%, **P = 0.0064 in the tibialis anterior; +35%, **P = 0.0042 in the gastrocnemius) in the exercised A17 OPMD mice. In the supraphysiological resistance overload protocol, we also observed an increased collagen deposition (×2, ****P < 0.0001) in the plantaris muscle of A17 OPMD mice which was associated with larger muscle mass (×2, ****P < 0.0001) and fibre cross sectional area (×2, ***P = 0.0007) and increased absolute maximal force (×2, ****P < 0.0001) as well as a reduction in PABPN1 aggregate number (-16%, ****P < 0.0001).
Conclusions: Running exercise and mechanical overload led to very different outcome in skeletal muscles of A17 mice. Both types of exercise enhanced collagen deposition but while the running protocol increased aggregates, the OVL reduced them. More importantly OVL reversed muscle atrophy and maximal force in the A17 mice. Our study performed in a relevant model gives an indication of the effect of different types of exercise on OPMD muscle which should be further evaluated in humans for future recommendations as a part of the lifestyle of individuals with OPMD.
Keywords: Atrophy; Exercise; Fibrosis; OPMD; PAPBN1; Skeletal muscle.
© 2024 The Author(s). Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Brais B, Bouchard J‐P, Xie Y‐G, Rochefort DL, Chrétien N, Tomé FMS, et al. Short GCG expansions in the PABP2 gene cause oculopharyngeal muscular dystrophy. Nat Genet 1998;18:164–167. - PubMed
-
- Tomé FM, Fardeau M. Nuclear inclusions in oculopharyngeal dystrophy. Acta Neuropathol 1980;49:85–87. - PubMed
-
- DiPietro L, Dziura J, Yeckel CW, Neufer PD. Exercise and improved insulin sensitivity in older women: Evidence of the enduring benefits of higher intensity training. J Appl Physiol 2006;100:142–149. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

