SGLT2 inhibitors and nephrolithiasis risk: a meta-analysis
- PMID: 39113274
- PMCID: PMC11997758
- DOI: 10.1093/ndt/gfae179
SGLT2 inhibitors and nephrolithiasis risk: a meta-analysis
Erratum in
-
Correction.Nephrol Dial Transplant. 2025 May 30;40(6):1261. doi: 10.1093/ndt/gfae219. Nephrol Dial Transplant. 2025. PMID: 39436739 Free PMC article. No abstract available.
Abstract
Background: Sodium-glucose co-transporter 2 (SGLT2) inhibitors are novel anti-diabetic medications with potential beneficial effects on cardiovascular and renal outcomes, metabolic parameters and body weight. In addition to the beneficial effects on renal function, including estimated glomerular filtration rate and reduction in proteinuria, recent studies have investigated the potential role of SGLT2 inhibitor (SGLT2i) therapy on nephrolithiasis development. Nephrolithiasis, a condition affecting almost 10% of the general population at least once during a lifetime, is a common disorder with considerable risk for acute and chronic kidney injury and relatively few effective therapeutic options.
Methods: We performed a literature search through multiple databases, including PubMed, Ovid MEDLINE, Web of Science, Scopus and Cochrane Library. We followed the systematic review and meta-analysis guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses. We included a total of 11 635 698 patients who experienced nephrolithiasis from six clinical trials in this meta-analysis study.
Results: In the pooled analysis, nephrolithiasis occurred in 1.27% of patients in the SGLT2i group (n = 739 197), compared with 1.56% of patients (n = 10 896 501) in the control arm (active control, placebo or no therapy). SGLT-2 inhibitor therapy has been associated with a lower risk for nephrolithiasis compared with placebo {odds ratio [OR] 0.61 [95% confidence interval (CI) 0.53-0.70], P < .00001} or active therapy such as glucagon-like peptide 1 and dipeptidyl peptidase 4 inhibitors [OR 0.66 (95% CI 0.47-0.93), P = .02].
Conclusion: We demonstrated a lower risk of nephrolithiasis with SGLT2i therapy compared with placebo or active control. Potential underlying mechanisms include osmotic diuresis leading to a reduction in the concentration of lithogenic substances, anti-inflammatory and anti-fibrotic effects and an increase in urine pH. There is a clear need for future large-scale randomized clinical trials evaluating such associations for better understanding.
Keywords: calcium oxalate; glucosuria; nephrolithiasis; osmotic diuresis; sodium–glucose co-transporter 2 inhibitors.
© The Author(s) 2024. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
K.R.T. is supported by National Institutes of Health research grants R01MD014712, U2CDK114886, UL1TR002319, U54DK083912, U01DK100846, OT2HL161847, UM1AI109568 and OT2OD032581 and Centers for Disease Control projects 75D301-21-P-12254 and 75D301-23-C-18264. She has also received investigator-initiated grant support from Travere Therapeutics, Bayer and the Doris Duke Charitable Foundation outside of the submitted work. She reports consultancy fees from AstraZeneca, Boehringer Ingelheim, Bayer, Eli Lilly, Novo Nordisk, Travere Therapeutics and Pfizer and speaker fees from Novo Nordisk. P.R. reports personal fees from Bayer, research support and personal fees from AstraZeneca and Novo Nordisk and personal fees from Astellas, Boehringer Ingelheim, Eli Lilly, MSD, Gilead and Sanofi. All fees were paid to the Steno Diabetes Center Copenhagen. The remaining authors declare no conflicts of interest.
Figures

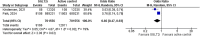
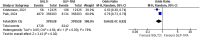
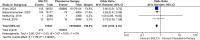
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

