T-cell specific in vivo gene delivery with DART-AAVs targeted to CD8
- PMID: 39113357
- PMCID: PMC11489536
- DOI: 10.1016/j.ymthe.2024.08.002
T-cell specific in vivo gene delivery with DART-AAVs targeted to CD8
Abstract
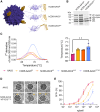
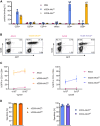
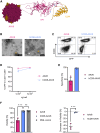
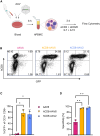
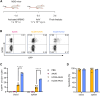
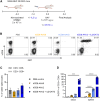
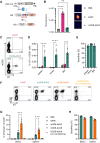
One of the biggest challenges for in vivo gene therapy are vectors mediating highly selective gene transfer into a defined population of therapy-relevant cells. Here we present DARPin-targeted AAVs (DART-AAVs) displaying DARPins specific for human and murine CD8. Insertion of DARPins into the GH2/GH3 loop of the capsid protein 1 (VP1) of AAV2 and AAV6 resulted in high selectivity for CD8-positive T cells with unimpaired gene delivery activity. Remarkably, the capsid core structure was unaltered with protruding DARPins detectable. In complex primary cell mixtures, including donor blood or systemic injections into mice, the CD8-targeted AAVs were by far superior to unmodified AAV2 and AAV6 in terms of selectivity, target cell viability, and gene transfer rates. In vivo, up to 80% of activated CD8+ T cells were hit upon a single vector injection into conditioned humanized or immunocompetent mice. While gene transfer rates decreased significantly under non-activated conditions, genomic modification selectively in CD8+ T cells was still detectable upon Cre delivery into indicator mice. In both mouse models, selectivity for CD8+ T cells was close to absolute with exceptional detargeting from liver. The CD8-AAVs described here expand strategies for immunological research and in vivo gene therapy options.
Keywords: AAV capsid engineering; CD8 targeting; CD8-AAV; DART-AAVs; T cell activation; T cell targeting; gene therapy; in vivo delivery; in vivo gene therapy; receptor targeting; targeted AAV; targeted gene therapy; viral vectors.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

References
-
- Jiang Z., Dalby P.A. Challenges in scaling up AAV-based gene therapy manufacturing. Trends Biotechnol. 2023;41:1268–1281. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

