Key learnings from the INBUILD trial in patients with progressive pulmonary fibrosis
- PMID: 39113425
- PMCID: PMC11311158
- DOI: 10.1177/17534666241266343
Key learnings from the INBUILD trial in patients with progressive pulmonary fibrosis
Abstract
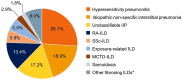
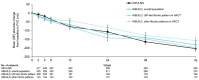
In a patient with interstitial lung disease (ILD) of known or unknown etiology other than idiopathic pulmonary fibrosis (IPF), progressive pulmonary fibrosis (PPF) is defined by worsening lung fibrosis on high-resolution computed tomography (HRCT), decline in lung function, and/or deterioration in symptoms. The INBUILD trial involved 663 patients with PPF who were randomized to receive nintedanib or placebo. The median exposure to trial medication was approximately 19 months. The INBUILD trial provided valuable learnings about the course of PPF and the efficacy and safety of nintedanib. The relative effect of nintedanib on reducing the rate of forced vital capacity decline was consistent across subgroups based on ILD diagnosis, HRCT pattern, and disease severity at baseline, and between patients who were and were not taking glucocorticoids or disease-modifying anti-rheumatic drugs and/or glucocorticoids at baseline. The adverse events most frequently associated with nintedanib were gastrointestinal, particularly diarrhea, but nintedanib was discontinued in only a minority of cases. The results of the INBUILD trial highlight the importance of prompt detection and treatment of PPF and the utility of nintedanib as a treatment option.
Keywords: clinical trial; disease progression; drug tolerance; interstitial; lung disease; pulmonary fibrosis.
Plain language summary
What did we find out from the INBUILD trial about progressive lung fibrosis?Lung fibrosis is a rare disease in which the lung tissue becomes scarred and hardened. This makes it more difficult for the lungs to inflate and for the lungs to exchange oxygen with the blood. In some patients, lung fibrosis gets worse over time. This is known as progressive lung fibrosis. In the INBUILD trial, researchers looked at the effects of a drug called nintedanib in patients with progressive lung fibrosis. In this trial, 663 patients were randomly allocated to receive either nintedanib or a placebo and then followed for approximately 19 months. The patients and the researchers did not know which patients were taking the active drug (nintedanib) and which patients were taking placebo. The results showed that the criteria used to find patients with progressive lung fibrosis to take part in the trial successfully identified patients whose disease would continue to worsen. These criteria were based on a decline in the volume (size) of the lungs, worsening symptoms such as shortness of breath, and worsening of changes seen on a scan of the chest. The trial results also showed that nintedanib slowed down loss of lung function and had a similar benefit in patients with different severities of disease at the start of the trial. The most common side-effects of nintedanib were gastrointestinal problems, particularly diarrhea, but most patients given nintedanib were able to cope with these side-effects without needing to stop treatment. Large trials like the INBUILD trial are important for helping us understand how diseases progress and in which patients particular drugs should be used.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures


References
-
- Flaherty KR, Wells AU, Cottin V, et al.. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med 2019; 381(18): 1718–1727. - PubMed
-
- Wells AU, Flaherty KR, Brown KK, et al.. Nintedanib in patients with progressive fibrosing interstitial lung diseases-subgroup analyses by interstitial lung disease diagnosis in the INBUILD trial: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Respir Med 2020; 8(5): 453–460. - PubMed
-
- George PM, Spagnolo P, Kreuter M, et al.. Progressive fibrosing interstitial lung disease: clinical uncertainties, consensus recommendations, and research priorities. Lancet Respir Med 2020; 8(9): 925–934. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

