Pharmacological inhibition of USP14 delays proteostasis-associated aging in a proteasome-dependent but foxo-independent manner
- PMID: 39113571
- PMCID: PMC11587835
- DOI: 10.1080/15548627.2024.2389607
Pharmacological inhibition of USP14 delays proteostasis-associated aging in a proteasome-dependent but foxo-independent manner
Abstract
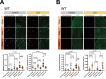
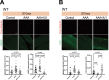
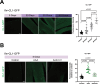
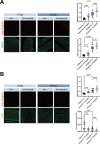
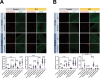
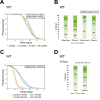
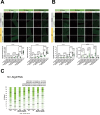
Aging is often accompanied by a decline in proteostasis, manifested as an increased propensity for misfolded protein aggregates, which are prevented by protein quality control systems, such as the ubiquitin-proteasome system (UPS) and macroautophagy/autophagy. Although the role of the UPS and autophagy in slowing age-induced proteostasis decline has been elucidated, limited information is available on how these pathways can be activated in a collaborative manner to delay proteostasis-associated aging. Here, we show that activation of the UPS via the pharmacological inhibition of USP14 (ubiquitin specific peptidase 14) using IU1 improves proteostasis and autophagy decline caused by aging or proteostatic stress in Drosophila and human cells. Treatment with IU1 not only alleviated the aggregation of polyubiquitinated proteins in aging Drosophila flight muscles but also extended the fly lifespan with enhanced locomotive activity via simultaneous activation of the UPS and autophagy. Interestingly, the effect of this drug disappeared when proteasomal activity was inhibited, but was evident upon proteostasis disruption by foxo mutation. Overall, our findings shed light on potential strategies to efficiently ameliorate age-associated pathologies associated with perturbed proteostasis.Abbreviations: AAAs: amino acid analogs; foxo: forkhead box, sub-group O; IFMs: indirect flight muscles; UPS: ubiquitin-proteasome system; USP14: ubiquitin specific peptidase 14.
Keywords: Autophagy; IU1; foxo; proteostasis; ubiquitin-proteasome system; ubiquitin-specific peptidase 14.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
