Pro-inflammatory activity of Cutibacterium acnes phylotype IA1 and extracellular vesicles: An in vitro study
- PMID: 39113601
- PMCID: PMC11605500
- DOI: 10.1111/exd.15150
Pro-inflammatory activity of Cutibacterium acnes phylotype IA1 and extracellular vesicles: An in vitro study
Abstract
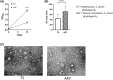
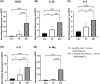
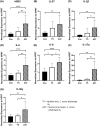
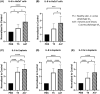
Acne is a chronic inflammatory skin condition that involves Cutibacterium acnes (C. acnes), which is classified into six main phylotypes (IA1, IA2, IB, IC, II and III). Acne development is associated with loss of C. acnes phylotype diversity, characterised by overgrowth of phylotype IA1 relative to other phylotypes. It was also shown that purified extracellular vesicles (EVs) secreted by C. acnes can induce an acne-like inflammatory response in skin models. We aimed to determine if the inflammatory profile of EVs secreted by C. acnes phylotype IA1 from an inflammatory acne lesion was different from C. acnes phylotype IA1 from normal skin, thus playing a direct role in the severity of inflammation. EVs were produced in vitro after culture of two clinical strains of C. acnes phylotype IA1, T5 from normal human skin and A47 from an inflammatory acne lesion, and then incubated with either human immortalised keratinocytes, HaCaT cells, or skin explants obtained from abdominoplasty. Subsequently, quantitative PCR (qPCR) was performed for human β-defensin 2 (hBD2), cathelicidin (LL-37), interleukin (IL)-1β, IL-6, IL-8, IL-17α and IL-36γ, and ELISA for IL-6, IL-8 and IL-17α. We found that EVs produced in vitro by C. acnes derived from inflammatory acne lesions significantly increased the pro-inflammatory cytokines and anti-microbial peptides at both transcriptional and protein levels compared with EVs derived from normal human skin. We show for the first time that C. acnes EVs from inflammatory acne play a crucial role in acne-associated inflammation in vitro and that C. acnes phylotype IA1 collected from inflammatory acne lesion and normal skin produce different EVs and inflammatory profiles in vitro.
Keywords: Cutibacterium acnes; acne; extracellular vesicles; inflammation; skin.
© 2024 The Author(s). Experimental Dermatology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that the research was conducted with funding from industrial sources.
Figures
References
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

