New systemic treatment options for advanced cholangiocarcinoma
- PMID: 39113642
- PMCID: PMC11449581
- DOI: 10.17998/jlc.2024.08.07
New systemic treatment options for advanced cholangiocarcinoma
Abstract
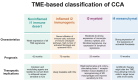
Cholangiocarcinoma (CCA) is a rare and aggressive cancer, mostly diagnosed at advanced or metastatic stage, at which point systemic treatment represents the only therapeutic option. Chemotherapy has been the backbone of advanced CCA treatment. More recently, immunotherapy has changed the therapeutic landscape, as immune checkpoint inhibitors have yielded the first improvement in survival and currently, the addition of either durvalumab or pembrolizumab to standard of care cisplatin plus gemcitabine represents the new first-line treatment option. However, the use of immunotherapy in subsequent lines has not demonstrated its efficacy and therefore, it is not approved, except for pembrolizumab in the selected microsatellite instability-high population. In addition, advances in comprehensive genomic profiling have led to the identification of targetable genetic alterations, such as isocitrate dehydrogenase 1 (IDH1), fibroblast growth factor receptor 2 (FGFR2), human epidermal growth factor receptor 2 (HER2), proto-oncogene B-Raf (BRAF), neurotrophic tropomyosin receptor kinase (NTRK), rearranged during transfection (RET), Kirsten rat sarcoma virus (KRAS), and mouse double minute 2 homolog (MDM2), thus favoring the development of a precision medicine approach in previously treated patients. Despite these advances, the use of molecularly driven agents is limited to a subgroup of patients. This review aims to provide an overview of the newly approved systemic therapies, the ongoing studies, and future research challenges in advanced CCA management.
Keywords: Cholangiocarcinoma; Immune checkpoint inhibitors; Immunotherapy; Systemic treatment; Targeted therapy.
Conflict of interest statement
Lorenza Rimassa received consulting fees from AbbVie, Astra- Zeneca, Basilea, Bayer, BMS, Elevar Therapeutics, Exelixis, Genenta, Hengrui, Incyte, Ipsen, IQVIA, Jazz Pharmaceuticals, MSD, Nerviano Medical Sciences, Roche, Servier, Taiho Oncology, Zymeworks. Lecture fees from AstraZeneca, Bayer, BMS, Guerbet, Incyte, Ipsen, Roche, Servier. Travel expenses from AstraZeneca. Research grants (to Institution) from Agios, AstraZeneca, BeiGene, Eisai, Exelixis, Fibrogen, Incyte, Ipsen, Lilly, MSD, Nerviano Medical Sciences, Roche, Servier, Taiho Oncology, TransThera Sciences, Zymeworks. All other authors declare no conflict of interest.
Figures

References
-
- Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: epidemiology and risk factors. Liver Int. 2019;39 Suppl 1:19–31. - PubMed
-
- Vithayathil M, Khan SA. Current epidemiology of cholangiocarcinoma in Western countries. J Hepatol. 2022;77:1690–1698. - PubMed
-
- Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20:663–673. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

