ISG15 accelerates acute kidney injury and the subsequent AKI-to-CKD transition by promoting TGFβR1 ISGylation
- PMID: 39113797
- PMCID: PMC11303071
- DOI: 10.7150/thno.95796
ISG15 accelerates acute kidney injury and the subsequent AKI-to-CKD transition by promoting TGFβR1 ISGylation
Abstract
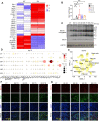
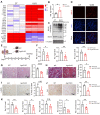
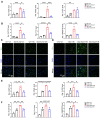
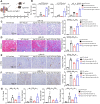
Rationale: Acute kidney injury (AKI) has substantial rates of mortality and morbidity, coupled with an absence of efficacious treatment options. AKI commonly transits into chronic kidney disease (CKD) and ultimately culminates in end-stage renal failure. The interferon-stimulated gene 15 (ISG15) level was upregulated in the kidneys of mice injured by ischemia-reperfusion injury (IRI), cisplatin, or unilateral ureteral obstruction (UUO), however, its role in AKI development and subsequent AKI-to-CKD transition remains unknown. Methods: Isg15 knockout (Isg15 KO) mice challenged with bilateral or unilateral IRI, cisplatin, or UUO were used to investigate its role in AKI. We established cellular models with overexpression or knockout of ISG15 and subjected them to hypoxia-reoxygenation, cisplatin, or transforming growth factor- β1 (TGF-β1) stimulation. Renal RNA-seq data obtained from AKI models sourced from public databases and our studies, were utilized to examine the expression profiles of ISG15 and its associated genes. Additionally, published single cell RNA-seq data from human kidney allograft biopsies and mouse IRI model were analyzed to investigate the expression patterns of ISG15 and the type I TGF-β receptor (TGFβR1). Western blotting, qPCR, co-immunoprecipitation, and immunohistochemical staining assays were performed to validate our findings. Results: Alleviated pathological injury and renal function were observed in Isg15 KO mice with IRI-, cisplatin-, or UUO-induced AKI and the following AKI-to-CKD transition. In hypoxia-reoxygenation, cisplatin or TGF-β1 treated HK-2 cells, knockout ISG15 reduced stimulus-induced cell fibrosis, while overexpression of ISG15 with modification capacity exacerbated cell fibrosis. Immunoprecipitation assays demonstrated that ISG15 promoted ISGylation of TGFβR1, and inhibited its ubiquitination. Moreover, knockout of TGFβR1 blocked ISG15's fibrosis-exacerbating effect in HK-2 cells, while overexpression of TGFβR1 abolished the renal protective effect of ISG15 knockout during IRI-induced kidney injury. Conclusions: ISG15 plays an important role in the development of AKI and subsequent AKI-to-CKD transition by promoting TGFβR1 ISGylation.
Keywords: AKI-to-CKD transition; ISG15; TGFβR1; acute kidney injury; fibrosis.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




References
-
- Zarbock A, Forni LG, Ostermann M, Ronco C, Bagshaw SM, Mehta RL. et al. Designing acute kidney injury clinical trials. Nat Rev Nephrol. 2024;20:137–46. - PubMed
-
- Pistolesi V, Regolisti G, Morabito S, Gandolfini I, Corrado S, Piotti G. et al. Contrast medium induced acute kidney injury: a narrative review. J Nephrol. 2018;31:797–812. - PubMed
-
- De Clercq L, Ailliet T, Schaubroeck H, Hoste EAJ. Acute and Chronic Cardiovascular Consequences of Acute Kidney Injury: A Systematic Review and Meta-Analysis. Cardiorenal Med. 2023;13:26–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

