Astrocyte-derived lactate aggravates brain injury of ischemic stroke in mice by promoting the formation of protein lactylation
- PMID: 39113798
- PMCID: PMC11303085
- DOI: 10.7150/thno.96375
Astrocyte-derived lactate aggravates brain injury of ischemic stroke in mice by promoting the formation of protein lactylation
Abstract
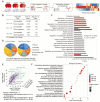
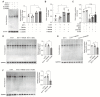
Aim: Although lactate supplementation at the reperfusion stage of ischemic stroke has been shown to offer neuroprotection, whether the role of accumulated lactate at the ischemia phase is neuroprotection or not remains largely unknown. Thus, in this study, we aimed to investigate the roles and mechanisms of accumulated brain lactate at the ischemia stage in regulating brain injury of ischemic stroke. Methods and Results: Pharmacological inhibition of lactate production by either inhibiting LDHA or glycolysis markedly attenuated the mouse brain injury of ischemic stroke. In contrast, additional lactate supplement further aggravates brain injury, which may be closely related to the induction of neuronal death and A1 astrocytes. The contributing roles of increased lactate at the ischemic stage may be related to the promotive formation of protein lysine lactylation (Kla), while the post-treatment of lactate at the reperfusion stage did not influence the brain protein Kla levels with neuroprotection. Increased protein Kla levels were found mainly in neurons by the HPLC-MS/MS analysis and immunofluorescent staining. Then, pharmacological inhibition of lactate production or blocking the lactate shuttle to neurons showed markedly decreased protein Kla levels in the ischemic brains. Additionally, Ldha specific knockout in astrocytes (Aldh1l1 CreERT2; Ldha fl/fl mice, cKO) mice with MCAO were constructed and the results showed that the protein Kla level was decreased accompanied by a decrease in the volume of cerebral infarction in cKO mice compared to the control groups. Furthermore, blocking the protein Kla formation by inhibiting the writer p300 with its antagonist A-485 significantly alleviates neuronal death and glial activation of cerebral ischemia with a reduction in the protein Kla level, resulting in extending reperfusion window and improving functional recovery for ischemic stroke. Conclusion: Collectively, increased brain lactate derived from astrocytes aggravates ischemic brain injury by promoting the protein Kla formation, suggesting that inhibiting lactate production or the formation of protein Kla at the ischemia stage presents new therapeutic targets for the treatment of ischemic stroke.
Keywords: brain injury; cerebral ischemia; glial activation; lactate; lactylation.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures






References
-
- Magistretti PJ, Allaman I. Lactate in the brain: from metabolic end-product to signalling molecule. Nat Rev Neurosci. 2018;19:235–49. - PubMed
-
- Allaman I, Magistretti P, Squire L, Berg D, Bloom F, du Lac S, Fundamental Neuroscience. Elsevier Amsterdam, The Netherlands. 2013.
-
- Michenfelder JD, Sundt TM Jr. Cerebral ATP and lactate levels in the squirrel monkey following occlusion of the middle cerebral artery. Stroke. 1971;2:319–26. - PubMed
-
- Wang J, Cui Y, Yu Z, Wang W, Cheng X, Ji W. et al. Brain Endothelial Cells Maintain Lactate Homeostasis and Control Adult Hippocampal Neurogenesis. Cell Stem Cell. 2019;25:754–67. e759. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

