Endosome-microautophagy targeting chimera (eMIATAC) for targeted proteins degradation and enhance CAR-T cell anti-tumor therapy
- PMID: 39113807
- PMCID: PMC11303074
- DOI: 10.7150/thno.98574
Endosome-microautophagy targeting chimera (eMIATAC) for targeted proteins degradation and enhance CAR-T cell anti-tumor therapy
Abstract
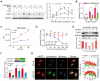
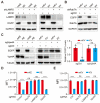
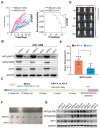
Rationale: Since oncogene expression products often exhibit upregulation or abnormally activated activity, developing a technique to regulate abnormal protein levels represent a viable approach for treating tumors and protein abnormality-related diseases. Methods: We first screened out eMIATAC components with high targeted degradation efficiency and explored the mechanism by which eMIATAC induced target protein degradation, and verified the degradation efficiency of the target protein by protein imprinting and flow cytometry. Next, we recombined eMIATAC with some controllable elements to verify the regulatable degradation performance of the target protein. Subsequently, we constructed eMIATAC that can express targeted degradation of AKT1 and verified its effect on GBM cell development in vitro and in vivo. Finally, we concatenated eMIATAC with CAR sequences to construct CAR-T cells with low BATF protein levels and verified the changes in their anti-tumor efficacy. Results: we developed a system based on the endosome-microautophagy-lysosome pathway for degrading endogenous proteins: endosome-MicroAutophagy TArgeting Chimera (eMIATAC), dependent on Vps4A instead of lysosomal-associated membrane protein 2A (LAMP2A) to bind to the chaperone Hsc70 and the protein of interest (POI). The complex was then transported to the lysosome by late endosomes, where degradation occurred similarly to microautophagy. The eMIATACs demonstrated accuracy, efficiency, reversibility, and controllability in degrading the target protein EGFP. Moreover, eMIATAC exhibited excellent performance in knocking down POI when targeting endogenous proteins in vivo and in vitro. Conclusions: The eMIATACs could not only directly knock down abnormal proteins for glioma treatment but also enhance the therapeutic effect of CAR-T cell therapy for tumors by knocking down T cell exhaustion-related proteins. The newly developed eMIATAC system holds promise as a novel tool for protein knockdown strategies. By enabling direct control over endogenous protein levels, eMIATAC has the potential to revolutionize treatment for cancer and genetic diseases.
Keywords: Autophagy degradation; Cancer therapy; Targeted protein degradation; eMIATAC.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Croce CM. Oncogenes and cancer. N Engl J Med. 2008;358(5):502–11. - PubMed
-
- Marazzi I, Greenbaum BD, Low DHP, Guccione E. Chromatin dependencies in cancer and inflammation. Nat Rev Mol Cell Biol. 2018;19(4):245–261. - PubMed
-
- Bykov VJN, Eriksson SE, Bianchi J, Wiman KG. Targeting mutant p53 for efficient cancer therapy. Nat Rev Cancer. 2018;18(2):89–102. - PubMed
-
- Zhan T, Rindtorff N, Betge J, Ebert MP, Boutros M. CRISPR/Cas9 for cancer research and therapy. Semin Cancer Biol. 2019;55:106–119. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

