Evaluation of fecal microbiota of late gestation sows in relation to pelvic organ prolapse risk
- PMID: 39113840
- PMCID: PMC11303877
- DOI: 10.3389/fmicb.2024.1384583
Evaluation of fecal microbiota of late gestation sows in relation to pelvic organ prolapse risk
Abstract
Introduction: Sow mortality in the U.S. swine industry has increased in recent years, for which pelvic organ prolapse (POP) is a major contributor, accounting for 21% of all sow mortality. Dysbiosis of microbial communities has been associated with disease and reproductive dysfunction in several species, and previous studies have shown changes in vaginal microbiota in sows with increased risk for POP during late gestation. However, there is insufficient knowledge surrounding the potential relationship between fecal microbiota and POP in sows. Therefore, the study objective was to identify differences in sow fecal microbiota and determine if fecal and vaginal microbial communities are correlated in relation to POP risk.
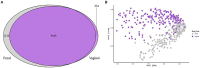
Methods: Sows were evaluated for POP risk using an established perineal scoring system, with a perineal score (PS) of 1 (PS1) presuming little to no risk of POP to a PS of 3 (PS3) presuming high risk of POP. In the current study, 2,864 sows were scored during gestation week 15, and 1.0%, 2.7%, and 23.4% of PS1, PS2, and PS3 sows, respectively, subsequently experienced POP. Fecal swabs (n = 215) were collected between gestation days 108-115, DNA was extracted, and 16S rRNA gene amplicon sequencing libraries were analyzed using mothur, phyloseq and SAS in reference to PS and POP outcome. Additionally, co-occurrence networks were constructed using CoNet to compare fecal and vaginal microbiota from the same cohort of sows and identify correlations between different taxa.
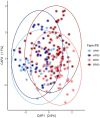
Results: Differences in fecal community composition (PERMANOVA; P < 0.05), structure (alpha diversity measurements; P < 0.05), and 13 individual operational taxonomic units (OTUs) were revealed between PS1 and PS3 assigned sows. No differences in fecal microbiota were detected as a result of POP outcome. However, the abundances of several taxa were correlated across sample collection sites, suggesting the fecal and vaginal microbial communities may be related to one another.
Discussion: Collectively, fewer differences in the fecal microbiota exist in sows with differing risk for POP compared to the vaginal microbiota, suggesting the vaginal microbiome may be more relevant in relation to POP outcome, although correlations between fecal and vaginal communities may provide insight for strategies to combat POP.
Keywords: fecal microbiota; pelvic organ prolapse; reproduction; sow; vaginal microbiota.
Copyright © 2024 Kiefer, Koester, Studer, Schmitz-Esser and Ross.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources

