Anaplastic thyroid cancer cell-secreted TGFβ1 plays a key role in inducing macrophage polarization of human monocytes
- PMID: 39113863
- PMCID: PMC11301286
- DOI: 10.62347/BHFA4606
Anaplastic thyroid cancer cell-secreted TGFβ1 plays a key role in inducing macrophage polarization of human monocytes
Abstract
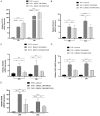
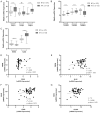
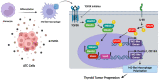
Anaplastic thyroid cancer (ATC) is a clinically aggressive form of undifferentiated thyroid cancer with limited treatment options. Tumor-associated macrophages (TAMs) constitute over 50% of ATC-infiltrating cells, and their presence is associated with a poor prognosis. We have previously shown that paracrine signals released by ATC cells induced pro-tumor M2-like polarization of human monocytes. However, which soluble factors derived from ATC cells drive monocyte activation, are largely unknown. In this study we investigated the participation of transforming growth factor β1 (TGFβ1) on the phenotype of macrophage activation induced by ATC cell-derived conditioned media (CM). THP-1 cells exposed to CM derived from ATC cells and recombinant human TGFβ1 induced M2-like macrophage polarization, showing high CD163 and Dectin1 expression. Moreover, we showed that TGFβ1 induced the messenger RNA (mRNA) and protein expression of the transcription factors SNAIL and SLUG. Accordingly, increased TGFβ1 secretion from ATC cells was confirmed by enzyme-linked immunosorbent assay (ELISA). Addition of SB431542, a TGFβ receptor inhibitor, significantly decreased the Dectin1, CD163, SNAIL and SLUG expression stimulated by ATC cell-derived CM. We validated the clinical significance of the expression of TGFβ ligands, their receptors, as well as SNAIL and SLUG in human ATC by analyzing public microarray datasets. We found that the expression of the main TGFβ ligands, TGFβ1 and TGFβ3, along with their receptors, TGFR1 and TGFR2, as well as SLUG, was significantly higher in human ATC tissue samples than in normal thyroid tissues. Our findings indicate that ATC cell-secreted TGFβ1 may play a key role in M2-like macrophage polarization of human monocytes and in the up-regulation of SNAIL and SLUG transcription factors. Thus, ours results uncovered a novel mechanism involved in the activation of TAMs by soluble factors released by ATC cells, which suggest potential therapeutic targets for ATC.
Keywords: Anaplastic thyroid cancer; M2-like macrophage polarization; TGFβ1; tumor-associated macrophages.
AJCR Copyright © 2024.
Conflict of interest statement
None.
Figures


References
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. - PubMed
-
- Molinaro E, Romei C, Biagini A, Sabini E, Agate L, Mazzeo S, Materazzi G, Sellari-Franceschini S, Ribechini A, Torregrossa L, Basolo F, Vitti P, Elisei R. Anaplastic thyroid carcinoma: from clinicopathology to genetics and advanced therapies. Nat Rev Endocrinol. 2017;13:644–660. - PubMed
-
- Bible KC, Kebebew E, Brierley J, Brito JP, Cabanillas ME, Clark TJ Jr, Di Cristofano A, Foote R, Giordano T, Kasperbauer J, Newbold K, Nikiforov YE, Randolph G, Rosenthal MS, Sawka AM, Shah M, Shaha A, Smallridge R, Wong-Clark CK. 2021 American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2021;31:337–386. - PMC - PubMed
-
- Subbiah V, Kreitman RJ, Wainberg ZA, Cho JY, Schellens JHM, Soria JC, Wen PY, Zielinski C, Cabanillas ME, Urbanowitz G, Mookerjee B, Wang D, Rangwala F, Keam B. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J. Clin. Oncol. 2018;36:7–13. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
