Glypican-3 deficiency in liver cancer upregulates MAPK/ERK pathway but decreases cell proliferation
- PMID: 39113871
- PMCID: PMC11301284
- DOI: 10.62347/TTNY4279
Glypican-3 deficiency in liver cancer upregulates MAPK/ERK pathway but decreases cell proliferation
Abstract
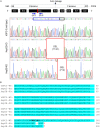
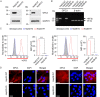
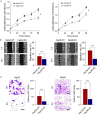
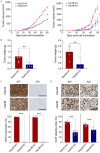
Glypican-3 (GPC3) is overexpressed in hepatocellular carcinomas and hepatoblastomas and represents an important therapeutic target but the biologic importance of GPC3 in liver cancer is unclear. To date, there are limited data characterizing the biological implications of GPC3 knockout (KO) in liver cancers that intrinsically express this target. Here, we report on the development and characterization of GPC3-KO liver cancer cell lines and compare to them to parental lines. GPC3-KO variants were established in HepG2 and Hep3B liver cancer cell lines using a lentivirus-mediated CRISPR/Cas9 system. We assessed the effects of GPC3 deficiency on oncogenic properties in vitro and in murine xenograft models. Downstream cellular signaling pathway changes induced by GPC3 deficiency were examined by RNAseq and western blot. To confirm the usefulness of the models for GPC3-targeted drug development, we evaluated the target engagement of a GPC3-selective antibody, GC33, conjugated to the positron-emitting zirconium-89 (89Zr) in subcutaneous murine xenografts of wild type (WT) and KO liver cancer cell lines. Deletion of GPC3 significantly reduced liver cancer cell proliferation, migration, and invasion compared to the parental cell lines. Additionally, the tumor growth of GPC3-KO liver cancer xenografts was significantly slower compared with control xenografts. RNA sequencing analysis also showed GPC3-KO resulted in a reduction in the expression of genes associated with cell cycle regulation, invasion, and migration. Specifically, we observed the downregulation of components in the AKT/NFκB/WNT signaling pathways and of molecules related to cell cycle regulation with GPC3-KO. In contrast, pMAPK/ERK1/2 was upregulated, suggesting an adaptive compensatory response. KO lines demonstrated increased sensitivity to ERK (GDC09994), while AKT (MK2206) inhibition was more effective in WT lines. Using antibody-based positron emission tomography (immunoPET) imaging, we confirmed that 89Zr-GC33 accumulated exclusively in GPC3-expression xenografts but not in GPC3-KO xenografts with high tumor uptake and tumor-to-liver signal ratio. We show that GPC3-KO liver cancer cell lines exhibit decreased tumorigenicity and altered signaling pathways, including upregulated pMAPK/ERK1/2, compared to parental lines. Furthermore, we successfully distinguished between GPC3+ and GPC3- tumors using the GPC3-targeted immunoPET imaging agent, demonstrating the potential utility of these cell lines in facilitating GPC3-selective drug development.
Keywords: Hepatocellular carcinoma; MAPK/ERK pathway; codrituzumab; glypican-3; hepatoblastoma; invasion; migration; molecular imaging; proliferation; radiopharmaceutical therapy.
AJCR Copyright © 2024.
Conflict of interest statement
None.
Figures


References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. - PubMed
-
- Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
