TRIM27 promotes the Warburg effect and glioblastoma progression via inhibiting the LKB1/AMPK/mTOR axis
- PMID: 39113875
- PMCID: PMC11301283
- DOI: 10.62347/TKFV8564
TRIM27 promotes the Warburg effect and glioblastoma progression via inhibiting the LKB1/AMPK/mTOR axis
Abstract
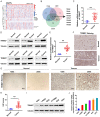
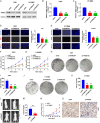
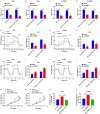
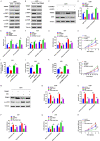
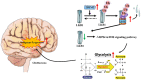
Altered protein ubiquitination is associated with cancer. The novel tripartite motif (TRIM) family of E3 ubiquitin ligases have been reported to play crucial roles in the development, growth, and metastasis of various tumors. The TRIM family member TRIM27 acts as a potential promoter of tumor development in a wide range of cancers. However, little is known regarding the biological features and clinical relevance of TRIM27 in glioblastoma (GBM). Here, we report findings of elevated TRIM27 expression in GBM tissues and GBM cell lines. Further functional analysis showed that TRIM27 deletion inhibited GBM cell growth both in vitro and in vivo. Furthermore, we found that TRIM27 promoted the growth of GBM cells by enhancing the Warburg effect. Additionally, the inactivation of the LKB1/AMPK/mTOR pathway was critical for the oncogenic effects of TRIM27 in GBM. Mechanistically, TRIM27 could directly bind to LKB1 and promote the ubiquitination and degradation of LKB1, which in turn enhanced the Warburg effect and GBM progression. Collectively, these data suggest that TRIM27 contributes to GNM pathogenesis by inhibiting the LKB1/AMPK/mTOR axis and may be a promising candidate as a potential diagnostic and therapeutic marker for patients with GBM.
Keywords: AMPK; Glioblastoma; LKB1; TRIM27; Warburg effect.
AJCR Copyright © 2024.
Conflict of interest statement
None.
Figures

References
-
- Ma R, Taphoorn MJB, Plaha P. Advances in the management of glioblastoma. J Neurol Neurosurg Psychiatry. 2021;92:1103–1111. - PubMed
-
- Campos B, Olsen LR, Urup T, Poulsen HS. A comprehensive profile of recurrent glioblastoma. Oncogene. 2016;35:5819–5825. - PubMed
-
- Le Rhun E, Preusser M, Roth P, Reardon DA, van den Bent M, Wen P, Reifenberger G, Weller M. Molecular targeted therapy of glioblastoma. Cancer Treat Rev. 2019;80:101896. - PubMed
-
- Popovic D, Vucic D, Dikic I. Ubiquitination in disease pathogenesis and treatment. Nat Med. 2014;20:1242–1253. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
