Roles and Mechanisms of Dopamine Receptor Signaling in Catecholamine Excess Induced Endothelial Dysfunctions
- PMID: 39113882
- PMCID: PMC11302566
- DOI: 10.7150/ijms.96550
Roles and Mechanisms of Dopamine Receptor Signaling in Catecholamine Excess Induced Endothelial Dysfunctions
Abstract
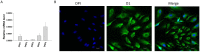
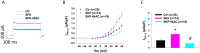
Endothelial dysfunction may contribute to pathogenesis of Takotsubo cardiomyopathy, but mechanism underlying endothelial dysfunction in the setting of catecholamine excess has not been clarified. The study reports that D1/D5 dopamine receptor signaling and small conductance calcium-activated potassium channels contribute to high concentration catecholamine induced endothelial cell dysfunction. For mimicking catecholamine excess, 100 μM epinephrine (Epi) was used to treat human cardiac microvascular endothelial cells. Patch clamp, FACS, ELISA, PCR, western blot and immunostaining analyses were performed in the study. Epi enhanced small conductance calcium-activated potassium channel current (ISK1-3) without influencing the channel expression and the effect was attenuated by D1/D5 receptor blocker. D1/D5 agonists mimicked the Epi effect, suggesting involvement of D1/D5 receptors in Epi effects. The enhancement of ISK1-3 caused by D1/D5 activation involved roles of PKA, ROS and NADPH oxidases. Activation of D1/D5 and SK1-3 channels caused a hyperpolarization, reduced NO production and increased ROS production. The NO reduction was membrane potential independent, while ROS production was increased by the hyperpolarization. ROS (H2O2) suppressed NO production. The study demonstrates that high concentration catecholamine can activate D1/D5 and SK1-3 channels through NADPH-ROS and PKA signaling and reduce NO production, which may facilitate vasoconstriction in the setting of catecholamine excess.
Keywords: Takotsubo syndrome; dopamine receptor; endothelial dysfunction, nitric oxide.; small conductance calcium-activated potassium channel.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures






Similar articles
-
Small conductance calcium-activated potassium channel contributes to stress induced endothelial dysfunctions.Microvasc Res. 2024 Sep;155:104699. doi: 10.1016/j.mvr.2024.104699. Epub 2024 Jun 18. Microvasc Res. 2024. PMID: 38901735
-
Catecholamine induces endothelial dysfunction via Angiotensin II and intermediate conductance calcium activated potassium channel.Biomed Pharmacother. 2024 Aug;177:116928. doi: 10.1016/j.biopha.2024.116928. Epub 2024 Jun 17. Biomed Pharmacother. 2024. PMID: 38889637
-
Dopamine D1/D5 Receptor Signaling Is Involved in Arrhythmogenesis in the Setting of Takotsubo Cardiomyopathy.Front Cardiovasc Med. 2022 Feb 4;8:777463. doi: 10.3389/fcvm.2021.777463. eCollection 2021. Front Cardiovasc Med. 2022. PMID: 35187102 Free PMC article.
-
Heteromerization of dopamine D2 receptors with dopamine D1 or D5 receptors generates intracellular calcium signaling by different mechanisms.Curr Opin Pharmacol. 2010 Feb;10(1):93-9. doi: 10.1016/j.coph.2009.09.011. Epub 2009 Nov 10. Curr Opin Pharmacol. 2010. PMID: 19897420 Free PMC article. Review.
-
Calcium-activated potassium channels and endothelial dysfunction: therapeutic options?Br J Pharmacol. 2009 Feb;156(4):545-62. doi: 10.1111/j.1476-5381.2009.00052.x. Epub 2009 Jan 29. Br J Pharmacol. 2009. PMID: 19187341 Free PMC article. Review.
Cited by
-
Recurrent Takotsubo Cardiomyopathy With Variable Patterns and Psychiatric Comorbidities: A Case Report and Comprehensive Literature Review.Clin Case Rep. 2025 Jul 10;13(7):e70635. doi: 10.1002/ccr3.70635. eCollection 2025 Jul. Clin Case Rep. 2025. PMID: 40655456 Free PMC article.
References
-
- Sato H. Tako-tsubo-like left ventricular dysfunction due to multivessel coronary spasm. Clinical aspects of myocardial injury: from ischemia to heart failure. Circulation. 1990. pp. 56–64.
-
- Sato H, Tateishi H, Uchida T, Dote K, Ishihara M, Kodama K, Clinical aspect of myocardial injury: from ischemia to heart failure. Kagaku Hyoronsha. 1990. pp. 55–64.
-
- Tsuchihashi K, Ueshima K, Uchida T, Oh-mura N, Kimura K, Owa M. et al. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. Angina Pectoris-Myocardial Infarction Investigations in Japan. J Am Coll Cardiol. 2001;38:11–8. - PubMed
-
- Pelliccia F, Kaski JC, Crea F, Camici PG. Pathophysiology of Takotsubo Syndrome. Circulation. 2017;135:2426–41. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

