Biological and Clinical Characteristics of Proximal Colon Cancer: Far from Its Anatomical Subsite
- PMID: 39113889
- PMCID: PMC11302569
- DOI: 10.7150/ijms.97574
Biological and Clinical Characteristics of Proximal Colon Cancer: Far from Its Anatomical Subsite
Abstract
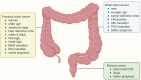
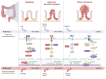
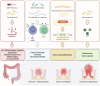
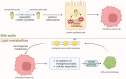
Colorectal cancer is a heterogeneous disease which can be divided into proximal colon cancer, distal colon cancer and rectal cancer according to the anatomical location of the tumor. Each anatomical location of colorectal cancer exhibits distinct characteristics in terms of incidence, clinical manifestations, molecular phenotypes, treatment, and prognosis. Notably, proximal colon cancer differs significantly from cancers of other anatomical subsites. An increasing number of studies have highlighted the presence of unique tumor biological characteristics in proximal colon cancer. Gaining a deeper understanding of these characteristics will facilitate accurate diagnosis and treatment approaches.
Keywords: Colorectal cancer; Gut microbiota; Proximal colon cancer; Right-sided colon cancer; Tumor biology.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
References
-
- Siegel RL, Wagle NS, Cercek A. et al. Colorectal cancer statistics, 2023. CA: A Cancer Journal for Clinicians. 2023;73:233–54. - PubMed
-
- Qu R, Ma Y, Zhang Z. et al. Increasing burden of colorectal cancer in China. Lancet Gastroenterol Hepatol. 2022;7:700. - PubMed
-
- Duraes LC, Steele SR, Valente MA. et al. Right colon, left colon, and rectal cancer have different oncologic and quality of life outcomes. Int J Colorectal Dis. 2022;37:939–48. - PubMed
-
- Yang SY, Cho MS, Kim NK. Difference between right-sided and left-sided colorectal cancers: from embryology to molecular subtype. Expert Review of Anticancer Therapy. 2018;18:351–8. - PubMed
-
- Araki K, Furuya Y, Kobayashi M. et al. Comparison of mucosal microvasculature between the proximal and distal human colon. J Electron Microsc (Tokyo) 1996;45:202–6. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

