Immune checkpoint inhibitor therapy‑related pneumonitis: How, when and why to diagnose and manage (Review)
- PMID: 39113908
- PMCID: PMC11304171
- DOI: 10.3892/etm.2024.12670
Immune checkpoint inhibitor therapy‑related pneumonitis: How, when and why to diagnose and manage (Review)
Abstract
Immune checkpoint inhibitor (ICI) therapy has revolutionized cancer treatment by enhancing the immune response against tumor cells. However, their influence on immune pathways can lead to immune-related adverse events such as pneumonitis, necessitating rapid diagnosis and management to prevent severe complications. These adverse events arise from the activation of the immune system by immunotherapeutic drugs, leading to immune-mediated inflammation and tissue damage in various organs and tissues throughout the body. The present review article discusses the pathophysiology, clinical presentation, diagnostic modalities and management strategies for ICI-related pneumonitis, emphasizing early recognition and tailored interventions. Future research endeavors should focus on elucidating the underlying mechanisms of pneumonitis and identifying predictive biomarkers to guide personalized treatment strategies in this evolving field of oncology.
Keywords: adverse events; diagnosis; immune checkpoint inhibitors; management; pneumonitis.
Copyright: © 2024 Lavalle et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

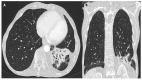

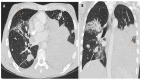
References
-
- Rapoport BL, Shannon VR, Cooksley T, Johnson DB, Anderson L, Blidner AG, Tintinger GR, Anderson R. Pulmonary toxicities associated with the use of immune checkpoint inhibitors: An update from the immuno-oncology subgroup of the neutropenia, infection & myelosuppression study group of the multinational association for supportive care in cancer. Front Pharmacol. 2021;12(743582) doi: 10.3389/fphar.2021.743582. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
