Mediation of radiation-induced bystander effect and epigenetic modification: The role of exosomes in cancer radioresistance
- PMID: 39114003
- PMCID: PMC11304029
- DOI: 10.1016/j.heliyon.2024.e34460
Mediation of radiation-induced bystander effect and epigenetic modification: The role of exosomes in cancer radioresistance
Abstract
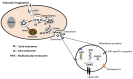
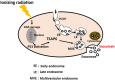
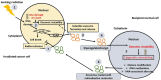
Exosomes are nano-sized extracellular vesicles produced by almost all mammalian cells. They play an important role in cell-to-cell communication by transferring biologically active molecules from the cell of origin to the recipient cells. Ionizing radiation influences exosome production and molecular cargo loading. In cancer management, ionizing radiation is a form of treatment that exerts its cancer cytotoxicity by induction of DNA damage and other alterations to the targeted tissue cells. However, normal bystander non-targeted cells may exhibit the effects of ionizing radiation, a phenomenon called radiation-induced bystander effect (RIBE). The mutual communication between the two groups of cells (targeted and non-targeted) via radiation-influenced exosomes enables the exchange of radiosensitive molecules. This facilitates indirect radiation exposure, leading, among other effects, to epigenetic remodeling and subsequent adaptation to radiation. This review discusses the role exosomes play in epigenetically induced radiotherapy resistance through the mediation of RIBE.
Keywords: Epigenetic changes; Exosomes; Ionizing radiation; Radiation-induced bystander effect; Radiotherapy resistance.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Non-targeted effects of radiation: a personal perspective on the role of exosomes in an evolving paradigm.Int J Radiat Biol. 2022;98(3):410-420. doi: 10.1080/09553002.2021.1980630. Epub 2021 Oct 21. Int J Radiat Biol. 2022. PMID: 34662248 Review.
-
Ionising Radiation Promotes Invasive Potential of Breast Cancer Cells: The Role of Exosomes in the Process.Int J Mol Sci. 2021 Oct 26;22(21):11570. doi: 10.3390/ijms222111570. Int J Mol Sci. 2021. PMID: 34769002 Free PMC article.
-
The Footprint of Exosomes in the Radiation-Induced Bystander Effects.Bioengineering (Basel). 2022 May 31;9(6):243. doi: 10.3390/bioengineering9060243. Bioengineering (Basel). 2022. PMID: 35735486 Free PMC article. Review.
-
Bystander autophagy mediated by radiation-induced exosomal miR-7-5p in non-targeted human bronchial epithelial cells.Sci Rep. 2016 Jul 15;6:30165. doi: 10.1038/srep30165. Sci Rep. 2016. PMID: 27417393 Free PMC article.
-
Exosomes in Cancer Radioresistance.Front Oncol. 2019 Sep 6;9:869. doi: 10.3389/fonc.2019.00869. eCollection 2019. Front Oncol. 2019. PMID: 31555599 Free PMC article. Review.
Cited by
-
Exosomal biomarkers: A novel frontier in the diagnosis of gastrointestinal cancers.World J Gastrointest Oncol. 2025 Apr 15;17(4):103591. doi: 10.4251/wjgo.v17.i4.103591. World J Gastrointest Oncol. 2025. PMID: 40235899 Free PMC article. Review.
-
Extracellular microvesicles/exosomes-magic bullets in horizontal transfer between cells of mitochondria and molecules regulating mitochondria activity.Stem Cells. 2025 Mar 10;43(3):sxae086. doi: 10.1093/stmcls/sxae086. Stem Cells. 2025. PMID: 39949038 Free PMC article. Review.
-
The Effect of Ionising Radiation on the Properties of Tumour-Derived Exosomes and Their Ability to Modify the Biology of Non-Irradiated Breast Cancer Cells-An In Vitro Study.Int J Mol Sci. 2025 Jan 4;26(1):376. doi: 10.3390/ijms26010376. Int J Mol Sci. 2025. PMID: 39796230 Free PMC article.
-
Systemic effects of 177Lu-DOTATATE therapy to patients with metastatic neuroendocrine tumors: mechanistic insights and role of exosome.Eur J Nucl Med Mol Imaging. 2025 Apr 23. doi: 10.1007/s00259-025-07291-2. Online ahead of print. Eur J Nucl Med Mol Imaging. 2025. PMID: 40263207
References
-
- Paolicelli R.C., Bergamini G., Rajendran L. Cell-to-cell communication by extracellular vesicles: focus on Microglia. Neuroscience. 2019;405:148–157. - PubMed
-
- van Niel G., D'Angelo G., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018;19(4):213–228. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

