Retinal debris triggers cytotoxic damage in cocultivated primary porcine RPE cells
- PMID: 39114482
- PMCID: PMC11303199
- DOI: 10.3389/fnins.2024.1401571
Retinal debris triggers cytotoxic damage in cocultivated primary porcine RPE cells
Abstract
Introduction: One of the most common causes of vision loss in the elderly population worldwide is age-related macular degeneration (AMD). Subsequently, the number of people affected by AMD is estimated to reach approximately 288 million by the year 2040. The aim of this study was to develop an ex vivo model that simulates various aspects of the complex AMD pathogenesis.
Methods: For this purpose, primary porcine retinal pigment epithelial cells (ppRPE) were isolated and cultured. One group was exposed to medium containing sodium iodate (NaIO3) to induce degeneration. The others were exposed to different supplemented media, such as bovine serum albumin (BSA), homogenized porcine retinas (HPR), or rod outer segments (ROOS) for eight days to promote retinal deposits. Then, these ppRPE cells were cocultured with porcine neuroretina explants for another eight days. To assess the viability of ppRPE cells, live/dead assay was performed at the end of the study. The positive RPE65 and ZO1 area was evaluated by immunocytochemistry and the expression of RLBP1, RPE65, and TJP1 was analyzed by RT-qPCR. Additionally, drusen (APOE), inflammation (ITGAM, IL6, IL8, NLRP3, TNF), oxidative stress (NFE2L2, SOD1, SOD2), and hypoxia (HIF1A) markers were investigated. The concentration of the inflammatory cytokines IL-6 and IL-8 was determined in medium supernatants from day 16 and 24 via ELISA.
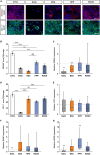
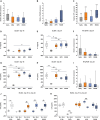
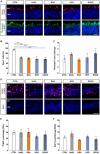
Results: Live/dead assay suggests that especially exposure to NaIO3 and HPR induced damage to ppRPE cells, leading in a significant ppRPE cell loss. All supplemented media resulted in decreased RPE-characteristic markers (RPE65; ZO-1) and gene expression like RLBP1 and RPE65 in the cultured ppRPE cells. Besides, some inflammatory, oxidative as well as hypoxic stress markers were altered in ppRPE cells cultivated with NaIO3. The application of HPR induced an enhanced APOE expression. Pre-exposure of the ppRPE cells led to a diminished number of cones in all supplemented media groups compared to controls.
Discussion: Overall, this novel coculture model represents an interesting initial approach to incorporating deposits into coculture to mimic AMD pathogenesis. Nevertheless, the effects of the media used need to be investigated in further studies.
Keywords: age-related macular degeneration; coculture system; deposits; neuroretina; retinal pigment epithelium (RPE).
Copyright © 2024 Wagner, Tsai, Reinehr, Theile, Dick and Joachim.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Impact of Primary RPE Cells in a Porcine Organotypic Co-Cultivation Model.Biomolecules. 2022 Jul 16;12(7):990. doi: 10.3390/biom12070990. Biomolecules. 2022. PMID: 35883547 Free PMC article.
-
Oxidative Stress-Induced Pentraxin 3 Expression Human Retinal Pigment Epithelial Cells is Involved in the Pathogenesis of Age-Related Macular Degeneration.Int J Mol Sci. 2019 Nov 29;20(23):6028. doi: 10.3390/ijms20236028. Int J Mol Sci. 2019. PMID: 31795454 Free PMC article.
-
Deficiency of thyroid hormone receptor protects retinal pigment epithelium and photoreceptors from cell death in a mouse model of age-related macular degeneration.Cell Death Dis. 2022 Mar 21;13(3):255. doi: 10.1038/s41419-022-04691-2. Cell Death Dis. 2022. PMID: 35314673 Free PMC article.
-
The Role of Retinal Pigment Epithelial Cells in Age-Related Macular Degeneration: Phagocytosis and Autophagy.Biomolecules. 2023 May 29;13(6):901. doi: 10.3390/biom13060901. Biomolecules. 2023. PMID: 37371481 Free PMC article. Review.
-
Not All Stressors Are Equal: Mechanism of Stressors on RPE Cell Degeneration.Front Cell Dev Biol. 2020 Nov 19;8:591067. doi: 10.3389/fcell.2020.591067. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33330470 Free PMC article. Review.
References
-
- Amin S., Chong N., Bailey T., Zhang J., Knupp C., Cheetham M., et al. (2004). Modulation of Sub-RPE deposits in vitro: A potential model for age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 45 1281–1288. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

