A network meta-analysis: evaluating the efficacy and safety of concurrent proton pump inhibitors and clopidogrel therapy in post-PCI patients
- PMID: 39114562
- PMCID: PMC11303300
- DOI: 10.3389/fcvm.2024.1385318
A network meta-analysis: evaluating the efficacy and safety of concurrent proton pump inhibitors and clopidogrel therapy in post-PCI patients
Abstract
Introduction: The objective of this research was to evaluate the risk of major adverse cardiovascular events (MACEs) associated with the use of various proton pump inhibitors (PPIs) in combination with clopidogrel in patients who underwent percutaneous coronary intervention (PCI).
Methods: To accomplish this, we analyzed data from randomized controlled trials and retrospective cohort studies sourced from key electronic databases. These studies specifically examined the effects of different PPIs, such as lansoprazole, esomeprazole, omeprazole, rabeprazole, and pantoprazole, when used in conjunction with clopidogrel on MACEs. The primary focus was on the differential impact of these PPIs, while the secondary focus was on the comparison of gastrointestinal (GI) bleeding events in groups receiving different PPIs with clopidogrel vs. a placebo group. This study's protocol was officially registered with INPLASY (INPLASY2024-2-0009).
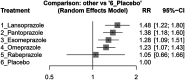
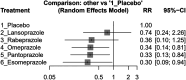
Results: We conducted a network meta-analysis involving 16 studies with a total of 145,999 patients. Our findings indicated that rabeprazole when combined with clopidogrel, had the lowest increase in MACE risk (effect size, 1.05, 95% CI: 0.66-1.66), while lansoprazole was associated with the highest risk increase (effect size, 1.48, 95% CI: 1.22-1.80). Esomeprazole (effect size, 1.28, 95% CI: 1.09-1.51), omeprazole (effect size, 1.23, 95% CI: 1.07-1.43), and pantoprazole (effect size, 1.38, 95% CI: 1.18-1.60) also significantly increased MACE risk. For the secondary outcome, esomeprazole (effect size, 0.30, 95% CI: 0.09-0.94), omeprazole (effect size, 0.34, 95% CI: 0.14-0.81), and pantoprazole (effect size, 0.33, 95% CI: 0.13-0.84) demonstrated an increased potential for GI bleeding prevention.
Conclusions: In conclusion, the combination of lansoprazole and clopidogrel was found to significantly elevate the risk of MACEs without offering GI protection in post-PCI patients. This study is the first network meta-analysis to identify the most effective regimen for the concurrent use of clopidogrel with individual PPIs.
Systematic review registration: https://inplasy.com/inplasy-2024-2-0009/, identifier (INPLASY2024-2-0009).
Keywords: clopidogrel; gastrointestinal (GI) bleeding; major adverse cardiovascular events (MACEs); post-percutaneous coronary intervention (PCI); proton pump inhibitors (PPIs).
© 2024 Ai, Chen, Kuo and Chang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Combination Use of Clopidogrel and Proton Pump Inhibitors Increases Major Adverse Cardiovascular Events in Patients With Coronary Artery Disease: A Meta-Analysis.J Cardiovasc Pharmacol Ther. 2017 Mar;22(2):142-152. doi: 10.1177/1074248416663647. Epub 2016 Aug 20. J Cardiovasc Pharmacol Ther. 2017. PMID: 27512080
-
Influence of individual proton pump inhibitors on clinical outcomes in patients receiving clopidogrel following percutaneous coronary intervention.Medicine (Baltimore). 2021 Dec 30;100(52):e27411. doi: 10.1097/MD.0000000000027411. Medicine (Baltimore). 2021. PMID: 34967346 Free PMC article.
-
No consistent evidence of differential cardiovascular risk amongst proton-pump inhibitors when used with clopidogrel: meta-analysis.Int J Cardiol. 2013 Aug 10;167(3):965-74. doi: 10.1016/j.ijcard.2012.03.085. Epub 2012 Mar 30. Int J Cardiol. 2013. PMID: 22464478
-
Changes in CYP2C19 enzyme activity evaluated by the [(13)C]-pantoprazole breath test after co-administration of clopidogrel and proton pump inhibitors following percutaneous coronary intervention and correlation to platelet reactivity.J Breath Res. 2016 Jan 27;10(1):017104. doi: 10.1088/1752-7155/10/1/017104. J Breath Res. 2016. PMID: 26815196 Clinical Trial.
-
Clinical relevance of clopidogrel-proton pump inhibitors interaction.World J Gastrointest Pharmacol Ther. 2015 May 6;6(2):17-21. doi: 10.4292/wjgpt.v6.i2.17. World J Gastrointest Pharmacol Ther. 2015. PMID: 25949846 Free PMC article. Review.
Cited by
-
Oral P2Y12 Inhibitors: Victims or Perpetrators? A Focused Review on Pharmacokinetic, Clinically Relevant Drug Interactions.Eur Cardiol. 2025 Jun 11;20:e17. doi: 10.15420/ecr.2025.12. eCollection 2025. Eur Cardiol. 2025. PMID: 40556646 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

