Trauma promotes heparan sulfate modifications and cleavage that disrupt homeostatic gene expression in microvascular endothelial cells
- PMID: 39114570
- PMCID: PMC11303185
- DOI: 10.3389/fcell.2024.1390794
Trauma promotes heparan sulfate modifications and cleavage that disrupt homeostatic gene expression in microvascular endothelial cells
Abstract
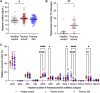
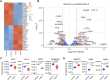
Introduction: Heparan sulfate (HS) in the vascular endothelial glycocalyx (eGC) is a critical regulator of blood vessel homeostasis. Trauma results in HS shedding from the eGC, but the impact of trauma on HS structural modifications that could influence mechanisms of vascular injury and repair has not been evaluated. Moreover, the effect of eGC HS shedding on endothelial cell (EC) homeostasis has not been fully elucidated. The objectives of this work were to characterize the impact of trauma on HS sulfation and determine the effect of eGC HS shedding on the transcriptional landscape of vascular ECs. Methods: Plasma was collected from 25 controls and 49 adults admitted to a level 1 trauma center at arrival and 24 h after hospitalization. Total levels of HS and angiopoietin-2, a marker of pathologic EC activation, were measured at each time point. Enzymatic activity of heparanase, the enzyme responsible for HS shedding, was determined in plasma from hospital arrival. Liquid chromatography-tandem mass spectrometry was used to characterize HS di-/tetrasaccharides in plasma. In vitro work was performed using flow conditioned primary human lung microvascular ECs treated with vehicle or heparinase III to simulate human heparanase activity. Bulk RNA sequencing was performed to determine differentially expressed gene-enriched pathways following heparinase III treatment. Results: We found that heparanase activity was increased in trauma plasma relative to controls, and HS levels at arrival were elevated in a manner proportional to injury severity. Di-/tetrasaccharide analysis revealed lower levels of 3-O-sulfated tetramers with a concomitant increase in ΔIIIS and ΔIIS disaccharides following trauma. Admission levels of total HS and specific HS sulfation motifs correlated with 24-h angiopoietin-2 levels, suggesting an association between HS shedding and persistent, pathological EC activation. In vitro pathway analysis demonstrated downregulation of genes that support cell junction integrity, EC polarity, and EC senescence while upregulating genes that promote cell differentiation and proliferation following HS shedding. Discussion: Taken together, our findings suggest that HS cleavage associated with eGC injury may disrupt homeostatic EC signaling and influence biosynthetic mechanisms governing eGC repair. These results require validation in larger, multicenter trauma populations coupled with in vivo EC-targeted transcriptomic and proteomic analyses.
Keywords: angiopoietin-2; endotheliopathy; glycocalyx; heparanase; sulfatase; sulfotransferase; transcriptome; vascular endothelium.
Copyright © 2024 Richter, Odum, Margaroli, Cardenas, Zheng, Tripathi, Wang, Arnold, Sanderson, Liu and Richter.
Conflict of interest statement
ZW was employed by Glycan Therapeutics Corp. JL is a founder and chief scientific officer for Glycan Therapeutics Corp. KA and JL own equity of Glycan Therapeutics Corp. KA is a founder for Glyco Discoveries, a subsidiary of Glycan Therapeutics Corp. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Targeting the "sweet spot" in septic shock - A perspective on the endothelial glycocalyx regulating proteins Heparanase-1 and -2.Matrix Biol Plus. 2021 Dec 2;12:100095. doi: 10.1016/j.mbplus.2021.100095. eCollection 2021 Dec. Matrix Biol Plus. 2021. PMID: 34917926 Free PMC article. Review.
-
Alterations in heparan sulfate proteoglycan synthesis and sulfation and the impact on vascular endothelial function.Matrix Biol Plus. 2022 Sep 7;16:100121. doi: 10.1016/j.mbplus.2022.100121. eCollection 2022 Dec. Matrix Biol Plus. 2022. PMID: 36160687 Free PMC article. Review.
-
Tie2 Activation Promotes Protection and Reconstitution of the Endothelial Glycocalyx in Human Sepsis.Thromb Haemost. 2019 Nov;119(11):1827-1838. doi: 10.1055/s-0039-1695768. Epub 2019 Sep 7. Thromb Haemost. 2019. PMID: 31493777
-
Heparan sulfate D-glucosaminyl 3-O-sulfotransferase-3B1 (HS3ST3B1) promotes angiogenesis and proliferation by induction of VEGF in acute myeloid leukemia cells.J Cell Biochem. 2015 Jun;116(6):1101-12. doi: 10.1002/jcb.25066. J Cell Biochem. 2015. PMID: 25536282
-
Endothelial glycocalyx shedding and vascular permeability in severely injured trauma patients.J Transl Med. 2015 Apr 12;13:117. doi: 10.1186/s12967-015-0481-5. J Transl Med. 2015. PMID: 25889764 Free PMC article.
Cited by
-
Shear Stress-Dependent Modulation of Endothelin B Receptor: The Role of Endothelial Glycocalyx Heparan Sulfate.Cells. 2025 Jul 16;14(14):1088. doi: 10.3390/cells14141088. Cells. 2025. PMID: 40710341 Free PMC article.
-
THE INTERACTION BETWEEN ANTITHROMBIN AND ENDOTHELIAL HEPARAN SULFATE MITIGATES PULMONARY THROMBOINFLAMMATION AFTER TRAUMA AND HEMORRHAGIC SHOCK.Shock. 2025 Apr 1;63(4):638-647. doi: 10.1097/SHK.0000000000002543. Epub 2025 Jan 23. Shock. 2025. PMID: 39847723
-
Injury induced endotheliopathy: overview, diagnosis, and management.Curr Opin Crit Care. 2025 Jun 1;31(3):237-243. doi: 10.1097/MCC.0000000000001239. Epub 2025 Jan 3. Curr Opin Crit Care. 2025. PMID: 39808442 Review.
References
-
- Abdullah S., Karim M., Legendre M., Rodriguez L., Friedman J., Cotton-Betteridge A., et al. (2021). Hemorrhagic shock and resuscitation causes glycocalyx shedding and endothelial oxidative stress preferentially in the lung and intestinal vasculature. Shock 56, 803–812. 10.1097/SHK.0000000000001764 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

