Cytosolic protein translation regulates cell asymmetry and function in early TCR activation of human CD8+ T lymphocytes
- PMID: 39114656
- PMCID: PMC11303187
- DOI: 10.3389/fimmu.2024.1411957
Cytosolic protein translation regulates cell asymmetry and function in early TCR activation of human CD8+ T lymphocytes
Erratum in
-
Corrigendum: Cytosolic protein translation regulates cell asymmetry and function in early TCR activation of human CD8+ T lymphocytes.Front Immunol. 2024 Oct 14;15:1500666. doi: 10.3389/fimmu.2024.1500666. eCollection 2024. Front Immunol. 2024. PMID: 39469710 Free PMC article.
Abstract
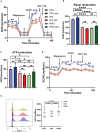
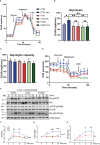
Introduction: CD8+ cytotoxic T lymphocytes (CTLs) are highly effective in defending against viral infections and tumours. They are activated through the recognition of peptide-MHC-I complex by the T-cell receptor (TCR) and co-stimulation. This cognate interaction promotes the organisation of intimate cell-cell connections that involve cytoskeleton rearrangement to enable effector function and clearance of the target cell. This is key for the asymmetric transport and mobilisation of lytic granules to the cell-cell contact, promoting directed secretion of lytic mediators such as granzymes and perforin. Mitochondria play a role in regulating CTL function by controlling processes such as calcium flux, providing the necessary energy through oxidative phosphorylation, and its own protein translation on 70S ribosomes. However, the effect of acute inhibition of cytosolic translation in the rapid response after TCR has not been studied in mature CTLs.
Methods: Here, we investigated the importance of cytosolic protein synthesis in human CTLs after early TCR activation and CD28 co-stimulation for the dynamic reorganisation of the cytoskeleton, mitochondria, and lytic granules through short-term chemical inhibition of 80S ribosomes by cycloheximide and 80S and 70S by puromycin.
Results: We observed that eukaryotic ribosome function is required to allow proper asymmetric reorganisation of the tubulin cytoskeleton and mitochondria and mTOR pathway activation early upon TCR activation in human primary CTLs.
Discussion: Cytosolic protein translation is required to increase glucose metabolism and degranulation capacity upon TCR activation and thus to regulate the full effector function of human CTLs.
Keywords: T cell activation; cell asymmetry; cytoskeleton; cytotoxic CD8+ T lymphocytes; immunological synapse; metabolism; mitochondria; protein translation.
Copyright © 2024 Gómez-Morón, Tsukalov, Scagnetti, Pertusa, Lozano-Prieto, Martínez-Fleta, Requena, Martín, Alfranca, Martin-Gayo and Martin-Cofreces.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

