Endometrial cancer treatment and outcomes in Argentina: ECHOS-A real-world study
- PMID: 39114806
- PMCID: PMC11305210
- DOI: 10.1016/j.gore.2024.101457
Endometrial cancer treatment and outcomes in Argentina: ECHOS-A real-world study
Abstract
Objective: Real-world data for patients with endometrial cancer (EC) are limited, particularly in Latin America. We present treatment pattern findings from ECHOS-A - Endometrial Cancer Health Outcomes Study in Argentina.
Materials and methods: A retrospective study using clinical data from privately insured patients with EC diagnosed from 2010 to 2019. Index (diagnosis proxy) was first date of an EC-related health term or treatment. Demographics, clinical characteristics, and FIGO staging were described. Disease progression and survival were assessed until study end, loss to follow-up, or death.
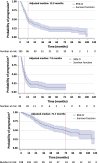
Results: Of 805 patients with EC, 77.4 % (n = 623/805) received any treatment and 22.6 % (n = 182/805) received none. Among those treated, 31.8 % (n = 198/623) had first-line (1L) systemic therapy, and 45.5 % (n = 90/198) proceeded to second-line (2L) therapy. Mean follow-up was 33.6 (SD 31.8) months. Of those receiving any treatment, 87.3 % (n = 544/623) had FIGO stage data (I, 62.9 %; II, 18.6 %; III, 13.6 %; IV, 5.0 %). Treatment by class in 1L and 2L, respectively, were platinum chemotherapy, 73.7 %, 36.7 %; non-platinum chemotherapy, 73.7 %, 62.2 %; immunotherapy, 1.0 %, 11.1 %; hormone therapy, 17.7 %, 26.7 %. Carboplatin/paclitaxel was the most frequent 1L (52.5 %) and 2L (14.4 %) regimen. Mean time to progression was 14.1 (SD 16.3) and 8.8 (SD 8.3) months in 1L and 2L, respectively. Adjusted 1- to 5-year risk of progression/death was 46.5-77.5 % and 65.0-86.2 % in 1L and 2L, respectively.
Conclusions: Approximately one-quarter of patients with EC received no treatment, and approximately two-thirds were not treated with 1L systemic therapy. Efforts to better understand the reasons for these treatment patterns are crucial for improving patient outcomes.
Keywords: Argentina; Endometrial cancer; Latin America; Overall survival; Progression-free survival; Real world.
© 2024 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: CS, PM, MC, and LJ are employees of and hold financial equities in GSK. GA, JQ, and TLNS are complementary employees of and do not hold financial equities in GSK. MCR reports receiving speaker fees and/or congress attendance support from AstraZeneca, GSK, and Roche. PS, NES, VAS, DO, and FC have no conflicts of interest to declare.
Figures


References
-
- Al-Talib A., Nezhat F., Tulandi T. The role of hysteroscopy in diagnosis and management of endometrial cancer. Gynecol. Surg. 2010;7:211–216.
LinkOut - more resources
Full Text Sources
