Ingestion of the Non-Nutritive Sweetener Erythritol, but Not Glucose, Enhances Platelet Reactivity and Thrombosis Potential in Healthy Volunteers-Brief Report
- PMID: 39114916
- PMCID: PMC11338701
- DOI: 10.1161/ATVBAHA.124.321019
Ingestion of the Non-Nutritive Sweetener Erythritol, but Not Glucose, Enhances Platelet Reactivity and Thrombosis Potential in Healthy Volunteers-Brief Report
Abstract
Background: Although artificial and non-nutritive sweeteners are widely used and generally recognized as safe by the US and European Union regulatory agencies, there have been no clinical trials to assess either long-term cardiovascular disease risks or short-term cardiovascular disease-relevant phenotypes. Recent studies report that fasting plasma levels of erythritol, a commonly used sweetener, are clinically associated with heightened incident cardiovascular disease risks and enhance thrombosis potential in vitro and in animal models. Effects of dietary erythritol on thrombosis phenotypes in humans have not been examined.
Methods: Using a prospective interventional study design, we tested the impact of erythritol or glucose consumption on multiple indices of stimulus-dependent platelet responsiveness in healthy volunteers (n=10 per group). Erythritol plasma levels were quantified with liquid chromatography tandem mass spectrometry. Platelet function at baseline and following erythritol or glucose ingestion was assessed via both aggregometry and analysis of granule markers released.
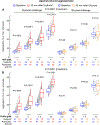
Results: Dietary erythritol (30 g), but not glucose (30 g), lead to a >1000-fold increase in erythritol plasma concentration (6480 [5930-7300] versus 3.75 [3.35-3.87] μmol/L; P<0.0001) and exhibited acute enhancement of stimulus-dependent aggregation responses in all subjects, agonists, and doses examined. Erythritol ingestion also enhanced stimulus-dependent release of the platelet dense granule marker serotonin (P<0.0001 for TRAP6 [thrombin activator peptide 6] and P=0.004 for ADP) and the platelet α-granule marker CXCL4 (C-X-C motif ligand-4; P<0.0001 for TRAP6 and P=0.06 for ADP). In contrast, glucose ingestion triggered no significant increases in stimulus-dependent release of either serotonin or CXCL4.
Conclusions: Ingestion of a typical quantity of the non-nutritive sweetener erythritol, but not glucose, enhances platelet reactivity in healthy volunteers, raising concerns that erythritol consumption may enhance thrombosis potential. Combined with recent large-scale clinical observational studies and mechanistic cell-based and animal model studies, the present findings suggest that discussion of whether erythritol should be reevaluated as a food additive with the Generally Recognized as Safe designation is warranted.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT04731363.
Keywords: models, animal; non-nutritive sweeteners; platelet aggregation; thrombosis.
Conflict of interest statement
S.L. Hazen and Z. Wang report being named as coinventors on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics, all unrelated to the subject and contents of this article. S.L. Hazen and Z. Wang also report having received royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Procter & Gamble and Cleveland HeartLab, Inc, a fully owned subsidiary of Quest Diagnostics. S.L. Hazen is a paid consultant at Zehna Therapeutics, has received research funds from Zehna Therapeutics, and is eligible to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics and therapeutics from Zehna Therapeutics. W.H.W. Tang reports being a consult at Sequana Medical A.G., Cardiol Therapeutics, Zehna Therapeutics, Genomics plc, Boston Scientific, WhiteSwell, Bristol Myers Squibb, Intellia Therapeutics, Alexion Pharmaceuticals, Alleviant Medical, CardiaTec Biosciences, Salubris Biotherapeutics, BioCardia, and has received honoraria from American Board of Internal Medicine, Belvoir Media Group, and Springer Nature. The other authors report no conflicts.
Figures

References
-
- Gardner C, Wylie-Rosett J, Gidding SS, Steffen LM, Johnson RK, Reader D, Lichtenstein AH. Nonnutritive sweeteners: current use and health perspectives: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation. 2012;126:509–519. doi: 10.1161/CIR.0b013e31825c42ee - DOI - PubMed
-
- Food and Drug Administration. GRAS Notice (GRN) No. 789. (2018). Available at: https://www.fda.gov/media/132946/download. 2018. Accessed 02/2024.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

