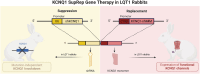
KCNQ1 suppression-replacement gene therapy in transgenic rabbits with type 1 long QT syndrome
- PMID: 39115049
- PMCID: PMC11439107
- DOI: 10.1093/eurheartj/ehae476
KCNQ1 suppression-replacement gene therapy in transgenic rabbits with type 1 long QT syndrome
Abstract
Background and aims: Type 1 long QT syndrome (LQT1) is caused by pathogenic variants in the KCNQ1-encoded Kv7.1 potassium channels, which pathologically prolong ventricular action potential duration (APD). Herein, the pathologic phenotype in transgenic LQT1 rabbits is rescued using a novel KCNQ1 suppression-replacement (SupRep) gene therapy.
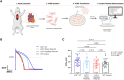
Methods: KCNQ1-SupRep gene therapy was developed by combining into a single construct a KCNQ1 shRNA (suppression) and an shRNA-immune KCNQ1 cDNA (replacement), packaged into adeno-associated virus serotype 9, and delivered in vivo via an intra-aortic root injection (1E10 vg/kg). To ascertain the efficacy of SupRep, 12-lead electrocardiograms were assessed in adult LQT1 and wild-type (WT) rabbits and patch-clamp experiments were performed on isolated ventricular cardiomyocytes.
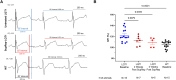
Results: KCNQ1-SupRep treatment of LQT1 rabbits resulted in significant shortening of the pathologically prolonged QT index (QTi) towards WT levels. Ventricular cardiomyocytes isolated from treated LQT1 rabbits demonstrated pronounced shortening of APD compared to LQT1 controls, leading to levels similar to WT (LQT1-UT vs. LQT1-SupRep, P < .0001, LQT1-SupRep vs. WT, P = ns). Under β-adrenergic stimulation with isoproterenol, SupRep-treated rabbits demonstrated a WT-like physiological QTi and APD90 behaviour.
Conclusions: This study provides the first animal-model, proof-of-concept gene therapy for correction of LQT1. In LQT1 rabbits, treatment with KCNQ1-SupRep gene therapy normalized the clinical QTi and cellular APD90 to near WT levels both at baseline and after isoproterenol. If similar QT/APD correction can be achieved with intravenous administration of KCNQ1-SupRep gene therapy in LQT1 rabbits, these encouraging data should compel continued development of this gene therapy for patients with LQT1.
Keywords: AAV9; Gene therapy; KCNQ1; Long QT syndrome; Transgenic LQT1 rabbits.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

