Eco-friendly Regioselective Synthesis, Biological Evaluation of Some New 5-acylfunctionalized 2-(1H-pyrazol-1-yl)thiazoles as Potential Antimicrobial and Anthelmintic Agents
- PMID: 39115105
- PMCID: PMC11564866
- DOI: 10.1002/open.202400142
Eco-friendly Regioselective Synthesis, Biological Evaluation of Some New 5-acylfunctionalized 2-(1H-pyrazol-1-yl)thiazoles as Potential Antimicrobial and Anthelmintic Agents
Abstract
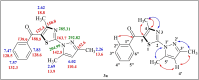
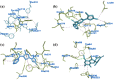
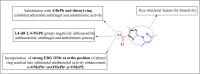
The present study describes an eco-friendly NBS-assisted regioselective synthesis of new 5-acylfunctionalized 5-acylfunctionalized 2-(1H-pyrazol-1-yl)thiazoles by condensation of 3,5-dimethyl-1H-pyrazole-1-carbothioamide with unsymmetrical 1,3-diketones under solvent-free conditions. The structural elucidation of the newly synthesized compounds was accomplished using various spectroscopic techniques viz. FTIR, NMR and mass spectrometry. All the newly synthesized compounds were examined for their in vitro antimicrobial potential against both pathogenic gram positive and gram negative bacterial and fungal species as well as anthelmintic activity against Pheretima posthuma earthworms. The results of antimicrobial activity revealed that all tested compounds 3 a-j showed excellent antimicrobial potential particularly against S. aureus. It was also observed that compounds 3 e and 3 i (MIC=62.5 μg/mL) showed greater potency against E. coli, whereas compounds 3 a and 3 h (MIC=50 μg/mL and 62.5 μg/mL) demonstrated better activity against P. aeruginosa and compound 3 i (MIC=62.5 μg/mL) exhibited superior activity against S. pyogenus when compared to standard drug Ampicillin (MIC=100μg/mL). Compound 3 e and 3 j revealed remarkable antifungal and anthelmintic activities. To find out binding interactions of target compounds with target proteins and pharmacokinetic parameters of the compounds, in silico investigations involving molecular docking studies and ADMET predictions were also performed.
Keywords: 2-(1H-pyrazol-1-yl)thiazoles; Solvent-free; anthelmintic; antimicrobial; multinuclear 2D NMR; structure activity relationship.
© 2024 The Authors. ChemistryOpen published by Wiley-VCH GmbH.
Conflict of interest statement
The author(s) declare no conflict of interest.
Figures





Similar articles
-
Synthesis of some thiazolyl aminobenzothiazole derivatives as potential antibacterial, antifungal and anthelmintic agents.J Enzyme Inhib Med Chem. 2011 Feb;26(1):22-8. doi: 10.3109/14756360903555258. Epub 2011 Jan 21. J Enzyme Inhib Med Chem. 2011. PMID: 21250821
-
Synthesis, characterization and biological studies of substituted quinozoline-4-(3H)-ones containing diazepine moiety.Ann Pharm Fr. 2014 Jan;72(1):51-8. doi: 10.1016/j.pharma.2013.11.001. Epub 2013 Dec 11. Ann Pharm Fr. 2014. PMID: 24438669
-
Synthesis, Molecular Docking, and Antimicrobial Evaluation of 2-(Substituted Amino)-N-(6-Substituted-1,3-Benzothiazol-2yl) Acetamide.Curr Drug Discov Technol. 2025;22(3):e200624231065. doi: 10.2174/0115701638299377240604112400. Curr Drug Discov Technol. 2025. PMID: 38910468
-
Antibacterial and antifungal pyrazoles based on different construction strategies.Eur J Med Chem. 2025 Jan 15;282:117081. doi: 10.1016/j.ejmech.2024.117081. Epub 2024 Nov 20. Eur J Med Chem. 2025. PMID: 39608204 Review.
-
Antimicrobial Potential of Natural Compounds of Zingiberaceae Plants and their Synthetic Analogues: A Scoping Review of In vitro and In silico Approaches.Curr Top Med Chem. 2024;24(13):1158-1184. doi: 10.2174/0115680266294573240328050629. Curr Top Med Chem. 2024. PMID: 38584545 Free PMC article.
References
-
- Sulis G., Sayood S., Katukoori S., Bollam N., George I., Yaeger L. H., Chavez M. A., Tetteh E., Yarrabelli S., Pulcini C., Harbarth S., Mertz D., Clin. Microbiol. Infect. 2022, 28, 1193–1202. - PubMed
-
- Guzman L., Cid C. P., Munoz E. G., Varas C. P., Cuadrado E. P., Duarte Y., Castro R. I., Nerio L. S., Maturana R. A., Asefa T., Echeverria J., Ramirez D., Doria O. F., Bioorg. Chem. 2021, 115, 105289. - PubMed
-
- Chandrasekhar D., Joseph C. M., parambil J. C., Murali S., Yahiya M., Shafeera K., Clin. Epidemiol. Glob. Health 2024, 28, 101499.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

