Interferon regulatory factor 8-driven reprogramming of the immune microenvironment enhances antitumor adaptive immunity and reduces immunosuppression in murine glioblastoma
- PMID: 39115195
- PMCID: PMC11630541
- DOI: 10.1093/neuonc/noae149
Interferon regulatory factor 8-driven reprogramming of the immune microenvironment enhances antitumor adaptive immunity and reduces immunosuppression in murine glioblastoma
Abstract
Background: Glioblastoma (GBM) has a highly immunosuppressive tumor immune microenvironment (TIME), largely mediated by myeloid-derived suppressor cells (MDSCs). Here, we utilized a retroviral replicating vector (RRV) to deliver Interferon Regulatory Factor 8 (IRF8), a master regulator of type 1 conventional dendritic cell (cDC1) development, in a syngeneic murine GBM model. We hypothesized that RRV-mediated delivery of IRF8 could "reprogram" intratumoral MDSCs into antigen-presenting cells and thereby restore T-cell responses.
Methods: Effects of RRV-IRF8 on survival and tumor growth kinetics were examined in the SB28 murine GBM model. The immunophenotype was analyzed by flow cytometry and gene expression assays. We assayed functional immunosuppression and antigen presentation by ex vivo T-cell-myeloid co-culture.
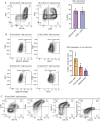
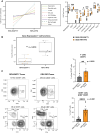
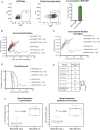
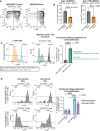
Results: Intratumoral injection of RRV-IRF8 in mice bearing intracerebral SB28 glioma significantly suppressed tumor growth and prolonged survival. RRV-IRF8 treated tumors exhibited significant enrichment of cDC1s and CD8+ T-cells. Additionally, myeloid cells derived from RRV-IRF8 tumors showed decreased expression of the immunosuppressive markers Arg1 and IDO1 and demonstrated reduced suppression of naïve T-cell proliferation in ex vivo co-culture, compared to controls. Furthermore, DCs from RRV-IRF8 tumors showed increased antigen presentation compared to those from control tumors. In vivo treatment with azidothymidine (AZT), a viral replication inhibitor, showed that IRF8 transduction in both tumor and non-tumor cells is necessary for survival benefit, associated with a reprogrammed, cDC1- and CD8 T-cell-enriched TIME.
Conclusions: Our results indicate that reprogramming of glioma-infiltrating myeloid cells by in vivo expression of IRF8 may reduce immunosuppression and enhance antigen presentation, achieving improved tumor control.
Keywords: IRF8; MDSC; RRV-mediated gene therapy; glioblastoma; immunosuppression.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
NK is Chief Scientific Officer at 4D Molecular Therapeutics.
Figures


Update of
-
IRF8-driven reprogramming of the immune microenvironment enhances anti-tumor adaptive immunity and reduces immunosuppression in murine glioblastoma.bioRxiv [Preprint]. 2024 Apr 3:2024.04.02.587608. doi: 10.1101/2024.04.02.587608. bioRxiv. 2024. Update in: Neuro Oncol. 2024 Dec 5;26(12):2272-2287. doi: 10.1093/neuonc/noae149. PMID: 38617245 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

