Engineering a Novel Probiotic Toolkit in Escherichia coli Nissle 1917 for Sensing and Mitigating Gut Inflammatory Diseases
- PMID: 39115381
- PMCID: PMC11334186
- DOI: 10.1021/acssynbio.4c00036
Engineering a Novel Probiotic Toolkit in Escherichia coli Nissle 1917 for Sensing and Mitigating Gut Inflammatory Diseases
Abstract
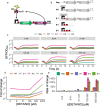
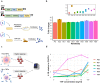
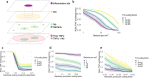
Inflammatory bowel disease (IBD) is characterized by chronic intestinal inflammation with no cure and limited treatment options that often have systemic side effects. In this study, we developed a target-specific system to potentially treat IBD by engineering the probiotic bacterium Escherichia coli Nissle 1917 (EcN). Our modular system comprises three components: a transcription factor-based sensor (NorR) capable of detecting the inflammation biomarker nitric oxide (NO), a type 1 hemolysin secretion system, and a therapeutic cargo consisting of a library of humanized anti-TNFα nanobodies. Despite a reduction in sensitivity, our system demonstrated a concentration-dependent response to NO, successfully secreting functional nanobodies with binding affinities comparable to the commonly used drug Adalimumab, as confirmed by enzyme-linked immunosorbent assay and in vitro assays. This newly validated nanobody library expands EcN therapeutic capabilities. The adopted secretion system, also characterized for the first time in EcN, can be further adapted as a platform for screening and purifying proteins of interest. Additionally, we provided a mathematical framework to assess critical parameters in engineering probiotic systems, including the production and diffusion of relevant molecules, bacterial colonization rates, and particle interactions. This integrated approach expands the synthetic biology toolbox for EcN-based therapies, providing novel parts, circuits, and a model for tunable responses at inflammatory hotspots.
Keywords: E. coli Nissle 1917 (EcN); IBD; TNFα; engineered probiotic; inflammation; nanobodies; nitric oxide.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Ng S. C.; Shi H. Y.; Hamidi N.; Underwood F. E.; Tang W.; Benchimol E. I.; Panaccione R.; Ghosh S.; Wu J. C. Y.; Chan F. K. L.; Sung J. J. Y.; Kaplan G. G. Worldwide Incidence and Prevalence of Inflammatory Bowel Disease in the 21st Century: A Systematic Review of Population-Based Studies. Lancet 2017, 390 (10114), 2769–2778. 10.1016/S0140-6736(17)32448-0. - DOI - PubMed
-
- Alatab S.; Sepanlou S. G.; Ikuta K.; Vahedi H.; Bisignano C.; Safiri S.; Sadeghi A.; Nixon M. R.; Abdoli A.; Abolhassani H.; Alipour V.; Almadi M. A. H.; Almasi-Hashiani A.; Anushiravani A.; Arabloo J.; Atique S.; Awasthi A.; Badawi A.; Baig A. A. A.; Bhala N.; Bijani A.; Biondi A.; Borzì A. M.; Burke K. E.; Carvalho F.; Daryani A.; Dubey M.; Eftekhari A.; Fernandes E.; Fernandes J. C.; Fischer F.; Haj-Mirzaian A.; Haj-Mirzaian A.; Hasanzadeh A.; Hashemian M.; Hay S. I.; Hoang C. L.; Househ M.; Ilesanmi O. S.; Jafari Balalami N.; James S. L.; Kengne A. P.; Malekzadeh M. M.; Merat S.; Meretoja T. J.; Mestrovic T.; Mirrakhimov E. M.; Mirzaei H.; Mohammad K. A.; Mokdad A. H.; Monasta L.; Negoi I.; Nguyen T. H.; Nguyen C. T.; Pourshams A.; Poustchi H.; Rabiee M.; Rabiee N.; Ramezanzadeh K.; Rawaf D. L.; Rawaf S.; Rezaei N.; Robinson S. R.; Ronfani L.; Saxena S.; Sepehrimanesh M.; Shaikh M. A.; Sharafi Z.; Sharif M.; Siabani S.; Sima A. R.; Singh J. A.; Soheili A.; Sotoudehmanesh R.; Suleria H. A. R.; Tesfay B. E.; Tran B.; Tsoi D.; Vacante M.; Wondmieneh A. B.; Zarghi A.; Zhang Z.-J.; Dirac M.; Malekzadeh R.; Naghavi M. The Global, Regional, and National Burden of Inflammatory Bowel Disease in 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5 (1), 17–30. 10.1016/S2468-1253(19)30333-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

