Pharmacogenetic Variants and Plasma Concentrations of Antiseizure Drugs: A Systematic Review and Meta-Analysis
- PMID: 39115847
- PMCID: PMC11310823
- DOI: 10.1001/jamanetworkopen.2024.25593
Pharmacogenetic Variants and Plasma Concentrations of Antiseizure Drugs: A Systematic Review and Meta-Analysis
Abstract
Importance: Precise estimation of a patient's drug metabolism capacity is important for antiseizure dose personalization.
Objective: To quantify the differences in plasma concentrations for antiseizure drugs associated with variants of genes encoding drug metabolizing enzymes.
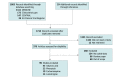
Data sources: PubMed, Clinicaltrialsregister.eu, ClinicalTrials.gov, International Clinical Trials Registry Platform, and CENTRAL databases were screened for studies from January 1, 1990, to September 30, 2023, without language restrictions.
Study selection: Two reviewers performed independent study screening and assessed the following inclusion criteria: appropriate genotyping was performed, genotype-based categorization into subgroups was possible, and each subgroup contained at least 3 participants.
Data extraction and synthesis: The Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines were followed for data extraction and subsequent quality, validity, and risk-of-bias assessments. The results from the included studies were pooled with random-effect meta-analysis.
Main outcomes and measures: Plasma concentrations of antiseizure drugs were quantified with the dose-normalized area under the concentration-time curve, the dose-normalized steady state concentration, or the concentrations after a single dose at standardized dose and sampling time. The ratio of the means was calculated by dividing the mean drug plasma concentrations of carriers and noncarriers of the pharmacogenetic variant.
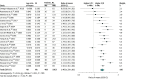
Results: Data from 98 studies involving 12 543 adult participants treated with phenytoin, valproate, lamotrigine, or carbamazepine were analyzed. Studies were mainly conducted within East Asian (69 studies) or White or European (15 studies) cohorts. Significant increases of plasma concentrations compared with the reference subgroup were observed for phenytoin, by 46% (95% CI, 33%-61%) in CYP2C9 intermediate metabolizers, 20% (95% CI, 17%-30%) in CYP2C19 intermediate metabolizers, and 39% (95% CI, 24%-56%) in CYP2C19 poor metabolizers; for valproate, by 12% (95% CI, 4%-20%) in CYP2C9 intermediate metabolizers, 12% (95% CI, 2%-24%) in CYP2C19 intermediate metabolizers, and 20% (95% CI, 2%-41%) in CYP2C19 poor metabolizers; and for carbamazepine, by 12% (95% CI, 3%-22%) in CYP3A5 poor metabolizers.
Conclusions and relevance: This systematic review and meta-analysis found that CYP2C9 and CYP2C19 genotypes encoding low enzymatic capacity were associated with a clinically relevant increase in phenytoin plasma concentrations, several pharmacogenetic variants were associated with statistically significant but only marginally clinically relevant changes in valproate and carbamazepine plasma concentrations, and numerous pharmacogenetic variants were not associated with statistically significant differences in plasma concentrations of antiseizure drugs.
Conflict of interest statement
Figures
Comment in
-
Antiseizure Drugs and Pharmacogenetics-Is There Signal in the Noise?JAMA Netw Open. 2024 Aug 1;7(8):e2425600. doi: 10.1001/jamanetworkopen.2024.25600. JAMA Netw Open. 2024. PMID: 39115850 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

