FDA-approved disulfiram inhibits the NLRP3 inflammasome by regulating NLRP3 palmitoylation
- PMID: 39116210
- PMCID: PMC11398858
- DOI: 10.1016/j.celrep.2024.114609
FDA-approved disulfiram inhibits the NLRP3 inflammasome by regulating NLRP3 palmitoylation
Abstract
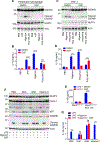
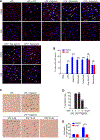
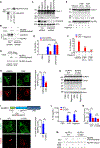
The NLRP3 inflammasome is dysregulated in autoinflammatory disorders caused by inherited mutations and contributes to the pathogenesis of several chronic inflammatory diseases. In this study, we discovered that disulfiram, a safe US Food and Drug Administration (FDA)-approved drug, specifically inhibits the NLRP3 inflammasome but not the NLRC4 or AIM2 inflammasomes. Disulfiram suppresses caspase-1 activation, ASC speck formation, and pyroptosis induced by several stimuli that activate NLRP3. Mechanistically, NLRP3 is palmitoylated at cysteine 126, a modification required for its localization to the trans-Golgi network and inflammasome activation, which was inhibited by disulfiram. Administration of disulfiram to animals inhibited the NLRP3, but not NLRC4, inflammasome in vivo. Our study uncovers a mechanism by which disulfiram targets NLRP3 and provides a rationale for using a safe FDA-approved drug for the treatment of NLRP3-associated inflammatory diseases.
Keywords: CP: Immunology; NLRP3; disulfiram; gasdermin D; inflammasome; palmitoylation.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

