Computational Design of Pore-Forming Peptides with Potent Antimicrobial and Anticancer Activities
- PMID: 39116273
- PMCID: PMC11345766
- DOI: 10.1021/acs.jmedchem.4c00912
Computational Design of Pore-Forming Peptides with Potent Antimicrobial and Anticancer Activities
Abstract
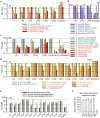
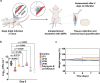
Peptides that form transmembrane barrel-stave pores are potential alternative therapeutics for bacterial infections and cancer. However, their optimization for clinical translation is hampered by a lack of sequence-function understanding. Recently, we have de novo designed the first synthetic barrel-stave pore-forming antimicrobial peptide with an identified function of all residues. Here, we systematically mutate the peptide to improve pore-forming ability in anticipation of enhanced activity. Using computer simulations, supported by liposome leakage and atomic force microscopy experiments, we find that pore-forming ability, while critical, is not the limiting factor for improving activity in the submicromolar range. Affinity for bacterial and cancer cell membranes needs to be optimized simultaneously. Optimized peptides more effectively killed antibiotic-resistant ESKAPEE bacteria at submicromolar concentrations, showing low cytotoxicity to human cells and skin model. Peptides showed systemic anti-infective activity in a preclinical mouse model of Acinetobacter baumannii infection. We also demonstrate peptide optimization for pH-dependent antimicrobial and anticancer activity.
Conflict of interest statement
The authors declare the following competing financial interest(s): R.V. and R.D. are inventors on an EU patent application filed by Masaryk University covering the peptides described in this paper.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

