C3 Glomerulopathy Recurs Early after Kidney Transplantation in Serial Biopsies Performed within the First 2 Years after Transplantation
- PMID: 39116277
- PMCID: PMC11321730
- DOI: 10.2215/CJN.0000000000000474
C3 Glomerulopathy Recurs Early after Kidney Transplantation in Serial Biopsies Performed within the First 2 Years after Transplantation
Abstract
Background: C3 glomerulopathy (C3G), which encompasses C3GN and dense deposit disease (DDD), results from dysregulation of the alternative complement pathway. Data on disease recurrence after kidney transplantation are limited, and details on histologic features of recurrent C3G are scarce. We aimed to evaluate C3G recurrence in the allograft, with a focus on histologic presentation and progression.
Methods: We retrospectively analyzed 18 patients with native kidney failure attributed to C3G (12 C3GN and six DDD), who received a kidney transplant from January 2016 to January 2023. Demographic, genetic, clinical, and histologic data were studied. The NanoString 770 genes PanCancer Immune Profiling Panel was used for transcriptomic analysis. Disease recurrence was the primary outcome.
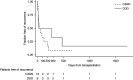
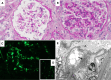
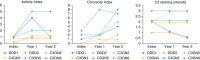
Results: During a median (interquartile range) follow-up period of 37 (18–56) months, C3G recurrence occurred in 16 (89%) patients (11 with C3GN and five with DDD) at a median (interquartile range) of 33 (13–141) days after transplantation. Over a third (38%) of recurrent cases were detected in protocol biopsies, and only 31% of patients presented with >300 mg/g of proteinuria. Recurrence in index biopsies was mainly established through a combination of immunofluorescence and electron microscopy findings, while it showed only subtle histologic alterations and no characteristic transcriptomic signals. Over time, histologic chronicity indices increased, but all the allografts were functioning at the end of follow-up. Patients with recurrence of C3GN and DDD showed overlapping immunofluorescence and electron microscopy findings and had similar recurrence rate and time to recurrence.
Conclusions: Most of the patients with native kidney failure attributed to C3G developed disease recurrence very early after kidney transplantation, usually with minimal proteinuria, mild histologic alterations, and favorable short-term allograft survival. Immunofluorescence and electron microscopy played a crucial role in detecting early, subclinical recurrence of C3GN and DDD, which showed significant overlapping features.
Conflict of interest statement
Disclosure forms, as provided by each author, are available with the online version of the article at
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

