Activated platelets retain and protect most of their factor XIII-A cargo from proteolytic activation and degradation
- PMID: 39116293
- PMCID: PMC11459904
- DOI: 10.1182/bloodadvances.2024012979
Activated platelets retain and protect most of their factor XIII-A cargo from proteolytic activation and degradation
Abstract
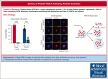
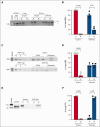
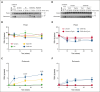
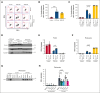
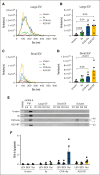
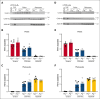
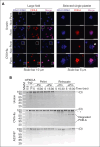
Platelet factor XIII-A (FXIII-A) is a major cytoplasmic protein (∼3% of total), representing ∼50% of total circulating FXIII. However, mobilization of FXIII-A during platelet activation is not well defined. To determine mechanisms mediating the retention vs release of platelet FXIII-A, platelets from healthy humans and mice (F13a1-/-, Fga-/-, Plg-/-, Stim1fl/flPf4-Cre, and respective controls) were stimulated with thrombin, convulxin plus thrombin, or calcium ionophore (A23187), in the absence or presence of inhibitors of transglutaminase activity, messenger RNA (mRNA) translation, microtubule rearrangement, calpain, and Rho GTPase. Platelet releasates and pellets were separated by (ultra)centrifugation. FXIII-A was detected by immunoblotting and immunofluorescence microscopy. Even after strong dual agonist (convulxin plus thrombin) stimulation of human platelets, >80% platelet FXIII-A remained associated with the platelet pellet. In contrast, essentially all tissue factor pathway inhibitor, another cytoplasmic protein in platelets, was released to the supernatant. Pellet-associated FXIII-A was not due to de novo synthesis via platelet F13A1 mRNA. The proportion of platelet FXIII-A retained by vs released from activated platelets was partly dependent on STIM1 signaling, microtubule rearrangement, calpain, and RhoA activation but did not depend on the presence of fibrinogen or plasminogen. Immunofluorescence microscopy confirmed the presence of considerable FXIII-A within the activated platelets. Although released FXIII-A was cleaved to FXIII-A∗ and could be degraded by plasmin, platelet-associated FXIII-A remained uncleaved. Retention of substantial platelet-derived FXIII-A by activated platelets and its reduced susceptibility to thrombin- and plasmin-mediated proteolysis suggest platelet FXIII-A is a protected pool with biological role(s) that differs from plasma FXIII.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

References
-
- Muszbek L, Bereczky Z, Bagoly Z, Komaromi I, Katona E. Factor XIII: a coagulation factor with multiple plasmatic and cellular functions. Physiol Rev. 2011;91(3):931–972. - PubMed
-
- Katona E E, Ajzner E, Toth K, Karpati L, Muszbek L. Enzyme-linked immunosorbent assay for the determination of blood coagulation factor XIII A-subunit in plasma and in cell lysates. J Immunol Methods. 2001;258(1-2):127–135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
