Minimally Invasive Injectable Gel for Local Immunotherapy of Liver and Gastric Cancer
- PMID: 39116306
- PMCID: PMC11481255
- DOI: 10.1002/advs.202405935
Minimally Invasive Injectable Gel for Local Immunotherapy of Liver and Gastric Cancer
Abstract
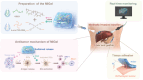
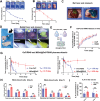
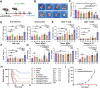
Local immunotherapy represents a promising solution for preventing tumor recurrence and metastasis post tumor surgical resection by eliminating residue tumor cells as well as eliciting tumor-specific immune responses. Minimally invasive surgery has become a mainstream surgical method worldwide due to its advantages of aesthetics and rapid postoperative recovery. Unfortunately, the currently reported local immunotherapy strategies are mostly designed to be used after open laparotomy, which go against the current surgical philosophy of minimally invasive therapy and is not suitable for clinical translation. Aiming at this problem, a minimally invasive injectable gel (MIGel) is herein reported loaded with immunotherapeutic agents for gastric and liver cancer postoperative treatment. The MIGel is formed by crosslinking between oxidized dextran (ODEX) and 4-arm polyethylene glycol hydroxylamine (4-arm PEG-ONH2) through oxime bonds, which can be injected through a clinic-used minimally invasive drainage tube and adhered tightly to the tissue. The loaded oxaliplatin (OxP) and resiquimod (R848) can be released constantly over two weeks and resulted in over 75% cure rate in orthotopic mouse gastric and liver cancer model. Collectively, a concept of minimally invasive local immunotherapy is proposed and MIGel is designed for local intraperitoneal cancer immunotherapy through minimally invasive surgery, with good clinical translation potential.
Keywords: cancer immunotherapy; gastric cancer; hydrogels; liver cancer; minimally invasive injection.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Injectable shear-thinning polylysine hydrogels for localized immunotherapy of gastric cancer through repolarization of tumor-associated macrophages.Biomater Sci. 2021 Sep 28;9(19):6597-6608. doi: 10.1039/d1bm01053k. Biomater Sci. 2021. PMID: 34582523
-
Comprehensive evaluation of biopolymer immune implants for peritoneal metastasis carcinoma therapy.J Control Release. 2023 Jan;353:289-302. doi: 10.1016/j.jconrel.2022.11.028. Epub 2022 Dec 2. J Control Release. 2023. PMID: 36403683
-
[Recognition of specialization of minimally invasive treatment for gastric cancer in the era of precision medicine].Zhonghua Wei Chang Wai Ke Za Zhi. 2017 Aug 25;20(8):847-851. Zhonghua Wei Chang Wai Ke Za Zhi. 2017. PMID: 28836240 Chinese.
-
Current status of surgical treatment of gastric cancer in the era of minimally invasive surgery in China: Opportunity and challenge.Int J Surg. 2016 Apr;28:45-50. doi: 10.1016/j.ijsu.2016.02.027. Epub 2016 Feb 13. Int J Surg. 2016. PMID: 26889972 Review.
-
Injectable Functional Biomaterials for Minimally Invasive Surgery.Adv Healthc Mater. 2020 Jul;9(13):e2000349. doi: 10.1002/adhm.202000349. Epub 2020 Jun 2. Adv Healthc Mater. 2020. PMID: 32484311 Review.
Cited by
-
A syringeable immunotherapeutic hydrogel enhances T cell immunity via in-situ activation of STING pathway for advanced breast cancer postoperative therapy.Front Immunol. 2025 Mar 19;16:1523436. doi: 10.3389/fimmu.2025.1523436. eCollection 2025. Front Immunol. 2025. PMID: 40176815 Free PMC article.
References
-
- a) Adams T. D., Gress R. E., Smith S. C., Halverson R. C., Simper S. C., Rosamond W. D., LaMonte M. J., Stroup A. M., Hunt S. C., N. Engl. J. Med. 2007, 357, 753; - PubMed
- b) Macdonald J. S., Smalley S. R., Benedetti J., Hundahl S. A., Estes N. C., Stemmermann G. N., Haller D. G., Ajani J. A., Gunderson L. L., Jessup J. M., Martenson J. A., N. Engl. J. Med. 2001, 345, 725; - PubMed
- c) Sakuramoto S., Sasako M., Yamaguchi T., Kinoshita T., Fujii M., Nashimoto A., Furukawa H., Nakajima T., Ohashi Y., Imamura H., Higashino M., Yamamura Y., Kurita A., Arai K., Grp A. G., N. Engl. J. Med. 2007, 357, 1810. - PubMed
-
- a) Nicholson R. I., Gee J. M. W., Harper M. E., Eur. J. Cancer 2001, 37, S9; - PubMed
- b) Lyman G. H., Giuliano A. E., Somerfield M. R., Benson A. B., Bodurka D. C., Burstein H. J., Benson A. B., Bodurka D. C., Burstein H. J., Cochran A. J., Cody H. S., Edge S. B., Galper S., Hayman J. A., Kim T. Y., Perkins C. L., Podoloff D. A., Sivasubramaniam V. H., Turner R. R., Wahl R., Weaver D. L., Wolff A. C., Winer E. P., J. Clin. Oncol. 2005, 23, 7703; - PubMed
- c) Si X.‐h., Song W.‐t., Chen X.‐s., Acta Polym. Sin. 2023, 54, 837.
-
- a) Jin Q., Liu Z., Chen Q., J. Controlled Release 2021, 329, 882; - PubMed
- b) Yang C., Blum N. T., Lin J., Qu J., Huang P., Sci. Bull. 2020, 65, 1489; - PubMed
- c) Chen Q., Wang C., Zhang X., Chen G., Hu Q., Li H., Wang J., Wen D., Zhang Y., Lu Y., Yang G., Jiang C., Wang J., Dotti G., Gu Z., Nat. Nanotechnol. 2019, 14, 89; - PubMed
- d) Phuengkham H., Song C., Lim Y. T., Adv. Mater. 2019, 31, 1903242; - PubMed
- e) Wang F., Xie M., Huang Y., Liu Y., Liu X., Zhu L., Zhu X., Guo Y., Zhang C., Angew. Chem., Int. Ed. 2024, 63, 2315282; - PubMed
- f) Xiao Z., Li Y., Xiong L., Liao J., Gao Y., Luo Y., Wang Y., Chen T., Yu D., Wang T., Zhang C., Chen Z.‐S., Adv. Sci. 2023, 10, 2302918; - PMC - PubMed
- g) Zhu Z.‐y., Song W.‐t., Chen X.‐s., Acta Polym. Sin. 2023, 54, 534.
-
- a) Ficarra V., Novara G., Artibani W., Cestari A., Galfano A., Graefen M., Guazzoni G., Guillonneau B., Menon M., Montorsi F., Patel V., Rassweiler J., Van Poppel H., European Urology 2009, 55, 1037; - PubMed
- b) Nieboer T. E., Johnson N., Lethaby A., Tavender E., Curr E., Garry R., van Voorst S., Mol B. W. J., Kluivers K. B., Cochrane Database of Systematic Reviews 2009; - PubMed
- c) Wakabayashi G., Cherqui D., Geller D. A., Buell J. E., Kaneko H., Han H. S., Asbun H., O'Rourke N., Tanabe M., Koffron A. J., Tsung A., Soubrane O., Machado M. A., Gayet B., Troisi R. I., Pessaux P., Van Dam R. M., Scatton O., Abu Hilal M., Belli G., Kwon C. H. D., Edwin B., Choi G. H., Aldrighetti L. A., Cai X., Clemy S., Chen K.‐H., Schoen M. R., Sugioka A., Tang C.‐N., et al., Ann. Surg. 2015, 261, 619. - PubMed
-
- a) Hu C., Long L., Cao J., Zhang S., Wang Y., Chem. Eng. J. 2021, 411, 128564;
- b) Liang Y., Li Z., Huang Y., Yu R., Guo B., ACS Nano 2021, 15, 7078; - PubMed
- c) Zhou L., Dai C., Fan L., Jiang Y., Liu C., Zhou Z., Guan P., Tian Y., Xing J., Li X., Luo Y., Yu P., Ning C., Tan G., Adv. Funct. Mater. 2021, 31, 2007457.
MeSH terms
Substances
Grants and funding
- 82203885/National Natural Science Foundation of China
- 22105199/National Natural Science Foundation of China
- 22222509/National Natural Science Foundation of China
- 22375198/National Natural Science Foundation of China
- 51833010/National Natural Science Foundation of China
- 52103194/National Natural Science Foundation of China
- 121522KYSB20200029/Bureau of International Cooperation Chinese Academy of Sciences
- 2023C043-4/Jilin Province Development and Reform Commission
- YDZJ202402077CXJD/Jilin Provincial International Cooperation Key Laboratory of Biomedical Polymers
- YDZJ202101ZYTS131/Jilin Province Science and Technology Development Plan
- 20220402037GH/Jilin Province Science and Technology Development Plan
- 21ZY09/Changchun Science and Technology Development Plan
- 2020232/Youth Innovation Promotion Association of Chinese Academy of Sciences
LinkOut - more resources
Full Text Sources
Medical
