Intranasal Multiepitope PD-L1-siRNA-Based Nanovaccine: The Next-Gen COVID-19 Immunotherapy
- PMID: 39116324
- PMCID: PMC11515909
- DOI: 10.1002/advs.202404159
Intranasal Multiepitope PD-L1-siRNA-Based Nanovaccine: The Next-Gen COVID-19 Immunotherapy
Abstract
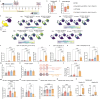
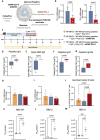
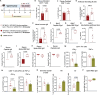
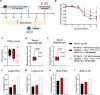
The first approved vaccines for human use against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are nanotechnology-based. Although they are modular, rapidly produced, and can reduce disease severity, the currently available vaccines are restricted in preventing infection, stressing the global demand for novel preventive vaccine technologies. Bearing this in mind, we set out to develop a flexible nanovaccine platform for nasal administration to induce mucosal immunity, which is fundamental for optimal protection against respiratory virus infection. The next-generation multiepitope nanovaccines co-deliver immunogenic peptides, selected by an immunoinformatic workflow, along with adjuvants and regulators of the PD-L1 expression. As a case study, we focused on SARS-CoV-2 peptides as relevant antigens to validate the approach. This platform can evoke both local and systemic cellular- and humoral-specific responses against SARS-CoV-2. This led to the secretion of immunoglobulin A (IgA), capable of neutralizing SARS-CoV-2, including variants of concern, following a heterologous immunization strategy. Considering the limitations of the required cold chain distribution for current nanotechnology-based vaccines, it is shown that the lyophilized nanovaccine is stable for long-term at room temperature and retains its in vivo efficacy upon reconstitution. This makes it particularly relevant for developing countries and offers a modular system adaptable to future viral threats.
Keywords: Dendritic cells; Intranasal; MHC class I and MHC class II peptides; Nanovaccines; PD‐1/PD‐L1 immune checkpoints; SARS‐CoV‐2; siRNA.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
R.S.‐F. is a Board Director at Teva Pharmaceutical Industries, Ltd. All other authors declare that they have no competing interests.
Figures


References
-
- Coronavirus (COVID‐19) Vaccinations, accessed: April, 2024, https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2.... - DOI
-
- Friedrichs S., Bowman D. M., Nat. Nanotechnol. 2021, 16, 362. - PubMed
-
- a) Barda N., Dagan N., Ben‐Shlomo Y., Kepten E., Waxman J., Ohana R., Hernán M. A., Lipsitch M., Kohane I., Netzer D., Reis B. Y., Balicer R. D., N. Engl. J. Med. 2021, 385, 1078; - PMC - PubMed
- b) Anderson E. J., Rouphael N. G., Widge A. T., Jackson L. A., Roberts P. C., Makhene M., Chappell J. D., Denison M. R., Stevens L. J., Pruijssers A. J., McDermott A. B., Flach B., Lin B. C., Doria‐Rose N. A., O'Dell S., Schmidt S. D., Corbett K. S., Swanson P. A., Padilla M., Neuzil K. M., Bennett H., Leav B., Makowski M., Albert J., Cross K., Edara V. V., Floyd K., Suthar M. S., Martinez D. R., Baric R., et al., N. Engl. J. Med. 2020, 383, 2427. - PMC - PubMed
-
- Uddin M. N., Roni M. A., Vaccines 2021, 9, 1033. - PubMed
MeSH terms
Substances
Grants and funding
- CF01-00014/"la Caixa" Foundation CaixaImpulse
- Fundação para a Ciência e Tecnologia-Ministério da Ciência
- UIDB/04138/2020/Tecnologia e Ensino Superior FCT-MCTES
- UIDP/04138/2020/Tecnologia e Ensino Superior FCT-MCTES
- PTDC/BTM-SAL/4350/2021/Tecnologia e Ensino Superior FCT-MCTES
- UTAP-EXPL/NPN/0041/2021/Tecnologia e Ensino Superior FCT-MCTES
- EXPL/MED-QUI/1316/2021/Tecnologia e Ensino Superior FCT-MCTES
- 591187(ImmuNovation)/European Research Council Proof of Concept
- PROF-18-682/Israel Cancer Research Fund
- LCF/TR/CD20/52700005/'la Caixa' Foundation
- 1969/18/Israel Science Foundation
- SAICTCOVID/72538/2020/Fundação para a Ciência e a Tecnologia
- ISIDORe project (ISID_c7f4) funded under HORIZON EUROPE
- 835227(3DBrainStrom)/ERC_/European Research Council/International
- 615808/MRA/Melanoma Research Alliance/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
