SMAD4 mutations causing Myhre syndrome are under positive selection in the male germline
- PMID: 39116879
- PMCID: PMC11444041
- DOI: 10.1016/j.ajhg.2024.07.006
SMAD4 mutations causing Myhre syndrome are under positive selection in the male germline
Abstract
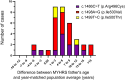
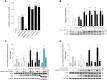
While it is widely thought that de novo mutations (DNMs) occur randomly, we previously showed that some DNMs are enriched because they are positively selected in the testes of aging men. These "selfish" mutations cause disorders with a shared presentation of features, including exclusive paternal origin, significant increase of the father's age, and high apparent germline mutation rate. To date, all known selfish mutations cluster within the components of the RTK-RAS-MAPK signaling pathway, a critical modulator of testicular homeostasis. Here, we demonstrate the selfish nature of the SMAD4 DNMs causing Myhre syndrome (MYHRS). By analyzing 16 informative trios, we show that MYHRS-causing DNMs originated on the paternally derived allele in all cases. We document a statistically significant epidemiological paternal age effect of 6.3 years excess for fathers of MYHRS probands. We developed an ultra-sensitive assay to quantify spontaneous MYHRS-causing SMAD4 variants in sperm and show that pathogenic variants at codon 500 are found at elevated level in sperm of most men and exhibit a strong positive correlation with donor's age, indicative of a high apparent germline mutation rate. Finally, we performed in vitro assays to validate the peculiar functional behavior of the clonally selected DNMs and explored the basis of the pathophysiology of the different SMAD4 sperm-enriched variants. Taken together, these data provide compelling evidence that SMAD4, a gene operating outside the canonical RAS-MAPK signaling pathway, is associated with selfish spermatogonial selection and raises the possibility that other genes/pathways are under positive selection in the aging human testis.
Keywords: TGF-β/BMP/activin pathway; de novo mutation; paternal age effect; rare disorder; selfish selection; spermatogonial stem cell.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

