Regulated induced proximity targeting chimeras-RIPTACs-A heterobifunctional small molecule strategy for cancer selective therapies
- PMID: 39116881
- PMCID: PMC11371387
- DOI: 10.1016/j.chembiol.2024.07.005
Regulated induced proximity targeting chimeras-RIPTACs-A heterobifunctional small molecule strategy for cancer selective therapies
Abstract
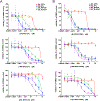
We describe a protein proximity inducing therapeutic modality called Regulated Induced Proximity Targeting Chimeras or RIPTACs: heterobifunctional small molecules that elicit a stable ternary complex between a target protein (TP) selectively expressed in tumor cells and a pan-expressed protein essential for cell survival. The resulting co-operative protein-protein interaction (PPI) abrogates the function of the essential protein, thus leading to death selectively in cells expressing the TP. This approach leverages differentially expressed intracellular proteins as novel cancer targets, with the advantage of not requiring the target to be a disease driver. In this chemical biology study, we design RIPTACs that incorporate a ligand against a model TP connected via a linker to effector ligands such as JQ1 (BRD4) or BI2536 (PLK1) or CDK inhibitors such as TMX3013 or dinaciclib. RIPTACs accumulate selectively in cells expressing the HaloTag-FKBP target, form co-operative intracellular ternary complexes, and induce an anti-proliferative response in target-expressing cells.
Keywords: Halda Therapeutics; RIPTAC; anticancer drugs; biotechnology; chemical biology; drug discovery; heterobifunctional molecules; oncology; protein proximity; ternary complex.
Copyright © 2024 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests C.M.C. is a shareholder and consultant to Halda Therapeutics, which supports research in his laboratory.
Figures

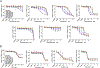




Update of
-
Regulated Induced Proximity Targeting Chimeras (RIPTACs): a Novel Heterobifunctional Small Molecule Therapeutic Strategy for Killing Cancer Cells Selectively.bioRxiv [Preprint]. 2023 Jan 2:2023.01.01.522436. doi: 10.1101/2023.01.01.522436. bioRxiv. 2023. Update in: Cell Chem Biol. 2024 Aug 15;31(8):1490-1502.e42. doi: 10.1016/j.chembiol.2024.07.005. PMID: 36711980 Free PMC article. Updated. Preprint.
Comment in
-
RIPTACs expand anticancer target space.Nat Rev Drug Discov. 2024 Oct;23(10):742. doi: 10.1038/d41573-024-00146-9. Nat Rev Drug Discov. 2024. PMID: 39251739 No abstract available.
References
-
- Bailey MH, Tokheim C, Porta-Pardo E, Sengupta S, Bertrand D, Weerasinghe A, Colaprico A, Wendl MC, Kim J, Reardon B, Ng PKS, Jeong KJ, Cao S, Wang Z, Gao J, Gao Q, Wang F, Liu EM, Mularoni L, … Karchin R (2018). Comprehensive Characterization of Cancer Driver Genes and Mutations. Cell, 173(2), 371–385.e18. 10.1016/j.cell.2018.02.060 - DOI - PMC - PubMed
-
- 2021 Clinical Development Success Rates 2011–2020. (2021). https://pharmaintelligence.informa.com/resources/product-content/2021-cl...
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

