Development of a 3-dimensional organotypic model with characteristics of peripheral sensory nerves
- PMID: 39116883
- PMCID: PMC11384078
- DOI: 10.1016/j.crmeth.2024.100835
Development of a 3-dimensional organotypic model with characteristics of peripheral sensory nerves
Abstract
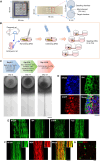
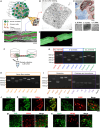
We developed a rat dorsal root ganglion (DRG)-derived sensory nerve organotypic model by culturing DRG explants on an organoid culture device. With this method, a large number of organotypic cultures can be produced simultaneously with high reproducibility simply by seeding DRG explants derived from rat embryos. Unlike previous DRG explant models, this organotypic model consists of a ganglion and an axon bundle with myelinated A fibers, unmyelinated C fibers, and stereo-myelin-forming nodes of Ranvier. The model also exhibits Ca2+ signaling in cell bodies in response to application of chemical stimuli to nerve terminals. Further, axonal transection increases the activating transcription factor 3 mRNA level in ganglia. Axons and myelin are shown to regenerate 14 days following transection. Our sensory organotypic model enables analysis of neuronal excitability in response to pain stimuli and tracking of morphological changes in the axon bundle over weeks.
Keywords: CP: Neuroscience; Schwann cells; ex vivo explant culture; microfluidic device; myelin; nerve regeneration; node of Ranvier; organotypic model; peripheral nervous system; peripheral neuropathy; sensory nerve.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
-
- Malheiro A., Harichandan A., Bernardi J., Seijas-Gamardo A., Konings G.F., Volders P.G.A., Romano A., Mota C., Wieringa P., Moroni L. 3D culture platform of human iPSCs-derived nociceptors for peripheral nerve modeling and tissue innervation. Biofabrication. 2021;14 doi: 10.1088/1758-5090/ac36bf. - DOI - PubMed
-
- Koyanagi M., Imai S., Iwamitsu Y., Matsumoto M., Saigo M., Moriya A., Ogihara T., Nakazato Y., Yonezawa A., Nakagawa S., et al. Cilostazol is an effective causal therapy for preventing paclitaxel-induced peripheral neuropathy by suppression of Schwann cell dedifferentiation. Neuropharmacology. 2021;188 doi: 10.1016/j.neuropharm.2021.108514. - DOI - PubMed
-
- Numata-Uematasu Y., Wakatsuki S., Kobayashi-Ujiie Y., Sakai K., Ichinohe N., Araki T. In vitro myelination using explant culture of dorsal root ganglia: An efficient tool for analyzing peripheral nerve differentiation and disease modeling. PLoS One. 2023;18 doi: 10.1371/journal.pone.0285897. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

