Key charged residues influence the amyloidogenic propensity of the helix-1 region of serum amyloid A
- PMID: 39117048
- PMCID: PMC11547331
- DOI: 10.1016/j.bbagen.2024.130690
Key charged residues influence the amyloidogenic propensity of the helix-1 region of serum amyloid A
Abstract
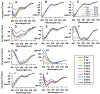
Increased plasma levels of serum amyloid A (SAA), an acute-phase protein that is secreted in response to inflammation, may lead to the accumulation of amyloid in various organs thereby obstructing their functions. Severe cases can lead to a systemic disorder called AA amyloidosis. Previous studies suggest that the N-terminal helix is the most amyloidogenic region of SAA. Moreover, computational studies implicated a significant role for Arg-1 and the residue-specific interactions formed during the fibrillization process. With a focus on the N-terminal region of helix-1, SAA1-13, mutational analysis was employed to interrogate the roles of the amino acid residues, Arg-1, Ser-5, Glu-9, and Asp-12. The truncated SAA1-13 fragment was systematically modified by substituting the key residues with alanine or uncharged but structurally similar amino acids. We monitored the changes in the amyloidogenic propensities, associated conformational markers, and morphology of the amyloids resulting from the mutation of SAA1-13. Mutating out Arg-1 resulted in much reduced aggregation propensity and a lack of detectable β-structures alluding to the importance of salt-bridge interactions involving Arg-1. Our data revealed that by systematically mutating the key amino acid residues, we can modulate the amyloidogenic propensity and alter the time-dependent conformational variation of the peptide. When the behaviors of each mutant peptide were analyzed, they provided evidence consistent with the aggregation pathway predicted by MD simulation studies. Here, we detail the important temporal molecular interactions formed by Arg-1 with Ser-5, Glu-9, and Asp-12 and discuss its mechanistic implications on the self-assembly of the helix-1 region of SAA.
Keywords: AA amyloidosis; Circular dichroism spectroscopy; Fourier transform infrared spectroscopy; Mechanism of aggregation; Serum amyloid a; Thioflavin T fluorescence assay.
Copyright © 2024 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no conflicts of interest.
Figures
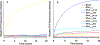


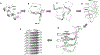
References
-
- Pettersson T, Konttinen YT, and Maury CP (2008) Treatment Strategies for Amyloid a Amyloidosis, Expert Opin Pharmaco 9, 2117–2128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

