Molecular phylogeny of the Lecudinoidea (Apicomplexa): A major group of marine gregarines with diverse shapes, movements and hosts
- PMID: 39117563
- PMCID: PMC11603279
- DOI: 10.1111/jeu.13053
Molecular phylogeny of the Lecudinoidea (Apicomplexa): A major group of marine gregarines with diverse shapes, movements and hosts
Abstract
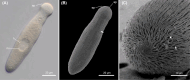
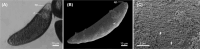
Gregarine apicomplexans are ubiquitous endosymbionts of invertebrate hosts. Despite their ecological and evolutionary importance, inferences about the phylogenetic relationships of major gregarine groups, such as the Lecudinidae and Urosporidae, have been hindered by vague taxonomic definitions and limited molecular and morphological data. In this study, we investigated five gregarine species collected from four families of polychaete hosts (Nereididae, Oenonidae, Hesionidae, and Phyllodocidae) using light microscopy (LM) and scanning electron microscopy (SEM). We also generated small subunit ribosomal DNA sequences from these species and conducted molecular phylogenetic analyses to elucidate the evolutionary relationships within the Lecudinoidea. Our results include new molecular and morphological data for two previously described species (Lecudina cf. platynereidis and Lecudina cf. arabellae), the discovery of a new species of Lecudina (L. oxydromus n. sp.), and the discovery of two novel species, namely Amplectina cordis n. gen. et. n. sp. and Sphinctocystis inclina n. sp. These two species exhibited unique shapes and movements, resembling those of urosporids but with a phylogenetic affinity to lecudinids, blurring the border between lecudinids and urosporids. Our study emphasizes the need for further investigations into this highly diverse group, which has achieved great success across multiple animal phyla with diverse shapes and movements.
Keywords: Lecudina; Gregarinasina; Urosporidae; molecular phylogeny; scanning electron microscopy.
© 2024 The Author(s). Journal of Eukaryotic Microbiology published by Wiley Periodicals LLC on behalf of International Society of Protistologists.
Figures





References
-
- Castresana, J. (2000). Selection of Conserved Blocks from Multiple Alignments for Their Use in Phylogenetic Analysis. Molecular Biology and Evolution, 17(4), 540–552. - PubMed
-
- Chakravarty, M.M. (1960) Systematic position of some genera and classifiction of the suborder Cephalina delage and Hérouard. Proceedings of Zoological Society, Kolkata, 12, 71–81.
-
- Clopton, R.E. (2009) Phylogenetic relationships, evolution, and systematic revision of the septate gregarines (Apicomplexa: Eugregarinorida: Septatorina). Comparative Parasitology, 76, 167–190.
-
- Darriba, D. , Taboada, G. , Doallo, R. & Posada, D. (2012) jModelTest 2: more models, new heuristics and parallel computing. Nature Methods, 9, 772. https://www.nature.com/articles/nmeth.2109 - PMC - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

