Preservation of retinal structure and function in two mouse models of inherited retinal degeneration by ONL1204, an inhibitor of the Fas receptor
- PMID: 39117629
- PMCID: PMC11310419
- DOI: 10.1038/s41419-024-06970-6
Preservation of retinal structure and function in two mouse models of inherited retinal degeneration by ONL1204, an inhibitor of the Fas receptor
Abstract
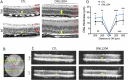
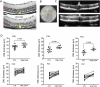
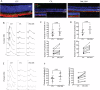
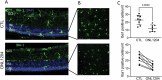
Due to the large number of genes and mutations that result in inherited retinal degenerations (IRD), there has been a paucity of therapeutic options for these patients. There is a large unmet need for therapeutic approaches targeting shared pathophysiologic pathways in a mutation-independent manner. The Fas receptor is a major activator and regulator of retinal cell death and inflammation in a variety of ocular diseases. We previously reported the activation of Fas-mediated photoreceptor (PR) cell death in two different IRD mouse models, rd10 and P23H, and demonstrated the protective effect of genetic Fas inhibition. The purpose of this study was to examine the effects of pharmacologic inhibition of Fas in these two models by intravitreal injection with a small peptide inhibitor of the Fas receptor, ONL1204. A single intravitreal injection of ONL1204 was given to one eye of rd10 mice at P14. Two intravitreal injections of ONL1204 were given to the P23H mice, once at P14 and again at 2-months of age. The fellow eyes were injected with vehicle alone. Fas activation, rate of PR cell death, retinal function, and the activation of immune cells in the retina were evaluated. In both rd10 and P23H mice, ONL1204 treatment resulted in decreased number of TUNEL (+) PRs, decreased caspase 8 activity, enhanced photoreceptor cell counts, and improved visual function compared with vehicle treated fellow eyes. Treatment with ONL1204 also reduced immune cell activation in the retinas of both rd10 and P23H mice. The protective effect of pharmacologic inhibition of Fas by ONL1204 in two distinct mouse models of retinal degeneration suggests that targeting this common pathophysiologic mechanism of cell death and inflammation represents a potential therapeutic approach to preserve the retina in patients with IRD, regardless of the genetic underpinning.
© 2024. The Author(s).
Conflict of interest statement
Two of the authors have relevant disclosures. AK is an employee of ONL Therapeutics, the company that makes ONL1204. DZ is an employee of the University of Michigan but is a co-founder of ONL Therapeutics with a personal financial interest in the company. Zacks also holds patents through the University of Michigan that are licensed to ONL Therapeutics.
Figures



Similar articles
-
Neuroprotection of photoreceptors by combined inhibition of both Fas and autophagy pathways in P23H mice.Cell Death Dis. 2025 Jul 1;16(1):469. doi: 10.1038/s41419-025-07793-9. Cell Death Dis. 2025. PMID: 40592891 Free PMC article.
-
Loss of Fas Receptor Function Preserves Photoreceptor Structure and Function in Two Mouse Models of Inherited Retinal Degeneration.Invest Ophthalmol Vis Sci. 2022 Sep 1;63(10):5. doi: 10.1167/iovs.63.10.5. Invest Ophthalmol Vis Sci. 2022. PMID: 36083588 Free PMC article.
-
A small peptide antagonist of the Fas receptor inhibits neuroinflammation and prevents axon degeneration and retinal ganglion cell death in an inducible mouse model of glaucoma.J Neuroinflammation. 2019 Sep 30;16(1):184. doi: 10.1186/s12974-019-1576-3. J Neuroinflammation. 2019. PMID: 31570110 Free PMC article.
-
Retinal Proteome Profiling of Inherited Retinal Degeneration Across Three Different Mouse Models Suggests Common Drug Targets in Retinitis Pigmentosa.Mol Cell Proteomics. 2024 Nov;23(11):100855. doi: 10.1016/j.mcpro.2024.100855. Epub 2024 Oct 9. Mol Cell Proteomics. 2024. PMID: 39389360 Free PMC article.
-
Retinal degeneration mutants in the mouse.Vision Res. 2002 Feb;42(4):517-25. doi: 10.1016/s0042-6989(01)00146-8. Vision Res. 2002. PMID: 11853768 Review.
Cited by
-
A Novel, Long-Acting, Small Molecule PKM2 Activator and Its Potential Broad Application Against Photoreceptor Degeneration.Transl Vis Sci Technol. 2025 Jul 1;14(7):26. doi: 10.1167/tvst.14.7.26. Transl Vis Sci Technol. 2025. PMID: 40742037 Free PMC article.
-
Neuroprotection of photoreceptors by combined inhibition of both Fas and autophagy pathways in P23H mice.Cell Death Dis. 2025 Jul 1;16(1):469. doi: 10.1038/s41419-025-07793-9. Cell Death Dis. 2025. PMID: 40592891 Free PMC article.
-
Anesthetic effects on electrophysiological responses across the visual pathway.Sci Rep. 2024 Nov 13;14(1):27825. doi: 10.1038/s41598-024-79240-2. Sci Rep. 2024. PMID: 39537872 Free PMC article.
-
Female sex hormones exacerbate retinal neurodegeneration.Sci Adv. 2025 Apr 11;11(15):eadr6211. doi: 10.1126/sciadv.adr6211. Epub 2025 Apr 11. Sci Adv. 2025. PMID: 40215317 Free PMC article.
-
Remote Preconditioning Provides Protection Against Retinal Cell Death From Retinal Detachment.Invest Ophthalmol Vis Sci. 2025 Feb 3;66(2):34. doi: 10.1167/iovs.66.2.34. Invest Ophthalmol Vis Sci. 2025. PMID: 39937497 Free PMC article.
References
-
- RetNet – Retinal information network. https://sph.uth.edu/retnet/. Accessed 30 Nov 2018.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

