Regulation of Zfp36 by ISGF3 and MK2 restricts the expression of inflammatory cytokines during necroptosis stimulation
- PMID: 39117638
- PMCID: PMC11310327
- DOI: 10.1038/s41419-024-06964-4
Regulation of Zfp36 by ISGF3 and MK2 restricts the expression of inflammatory cytokines during necroptosis stimulation
Abstract
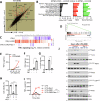
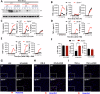
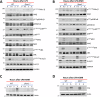
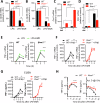
Necrosome activation following TLR- or cytokine receptor-signaling results in cell death by necroptosis which is characterized by the rupture of cell membranes and the consequent release of intracellular contents to the extracellular milieu. While necroptosis exacerbates various inflammatory diseases, the mechanisms through which the inflammatory responses are regulated are not clear. We show that the necrosome activation of macrophages results in an upregulation of various pathways, including the mitogen-activated protein kinase (MAPK) cascade, which results in an elevation of the inflammatory response and consequent expression of several cytokines and chemokines. Programming for this upregulation of inflammatory response occurs during the early phase of necrosome activation and proceeds independently of cell death but depends on the activation of the receptor-interacting protein kinase-1 (RipK1). Interestingly, necrosome activation also results in an upregulation of IFNβ, which in turn exerts an inhibitory effect on the maintenance of inflammatory response through the repression of MAPK-signaling and an upregulation of Zfp36. Activation of the interferon-induced gene factor-3 (ISGF3) results in the expression of ZFP36 (TTP), which induces the post-transcriptional degradation of mRNAs of various inflammatory cytokines and chemokines through the recognition of AU-rich elements in their 3'UTR. Furthermore, ZFP-36 inhibits IFNβ-, but not TNFα- induced necroptosis. Overall, these results reveal the molecular mechanism through which IFNβ, a pro-inflammatory cytokine, induces the expression of ZFP-36, which in turn inhibits necroptosis and halts the maintenance of the inflammatory response.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

