Light-responsive adipose-hypothalamus axis controls metabolic regulation
- PMID: 39117652
- PMCID: PMC11310318
- DOI: 10.1038/s41467-024-50866-0
Light-responsive adipose-hypothalamus axis controls metabolic regulation
Abstract
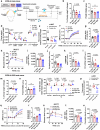
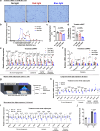
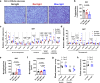
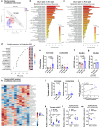
Light is fundamental for biological life, with most mammals possessing light-sensing photoreceptors in various organs. Opsin3 is highly expressed in adipose tissue which has extensive communication with other organs, particularly with the brain through the sympathetic nervous system (SNS). Our study reveals a new light-triggered crosstalk between adipose tissue and the hypothalamus. Direct blue-light exposure to subcutaneous white fat improves high-fat diet-induced metabolic abnormalities in an Opsin3-dependent manner. Metabolomic analysis shows that blue light increases circulating levels of histidine, which activates histaminergic neurons in the hypothalamus and stimulates brown adipose tissue (BAT) via SNS. Blocking central actions of histidine and denervating peripheral BAT blunts the effects of blue light. Human white adipocytes respond to direct blue light stimulation in a cell-autonomous manner, highlighting the translational relevance of this pathway. Together, these data demonstrate a light-responsive metabolic circuit involving adipose-hypothalamus communication, offering a potential strategy to alleviate obesity-induced metabolic abnormalities.
© 2024. The Author(s).
Conflict of interest statement
The authors declare the following competing interests: V.T., M.A.K., and N.R.N. are employees of BPGbio. The remaining authors declare no other competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
- R01 DK122808/DK/NIDDK NIH HHS/United States
- 903968/American Heart Association (American Heart Association, Inc.)
- R01DK122808/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)
- P30 DK036836/DK/NIDDK NIH HHS/United States
- S10OD028568/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- S10 OD028568/OD/NIH HHS/United States
- R01 DK133528/DK/NIDDK NIH HHS/United States
- P30DK036836/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)
- K01DK111714/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)
- K01 DK111714/DK/NIDDK NIH HHS/United States
- R01 DK132469/DK/NIDDK NIH HHS/United States
- R01DK102898/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)
- R01 DK102898/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources

