Paeonol alleviates ulcerative colitis by modulating PPAR-γ and nuclear factor-κB activation
- PMID: 39117680
- PMCID: PMC11310503
- DOI: 10.1038/s41598-024-68992-6
Paeonol alleviates ulcerative colitis by modulating PPAR-γ and nuclear factor-κB activation
Abstract
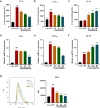
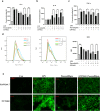
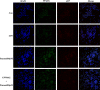
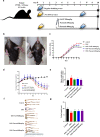
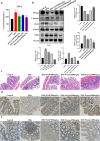
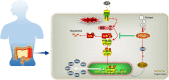
Ulcerative colitis (UC) is a chronic idiopathic inflammatory disease affecting the gastrointestinal tract. Although paeonol has been used for treating UC due to its anti-inflammatory and antioxidant effects, the underlying mechanisms remain unclear. In this study, we investigated the mechanisms of paeonol's action on UC by conducting in-vitro and in-vivo studies using NCM460 cells and RAW264.7 cells, and the DSS-induced mice colitis model. The in vitro studies demonstrate that paeonol exerts inhibitory effects on the activation of the NF-κB signaling pathway through upregulating PPARγ expression, thereby attenuating pro-inflammatory cytokine production, reducing reactive oxygen species levels, and promoting M2 macrophage polarization. These effects are significantly abrogated upon addition of the PPARγ inhibitor GW9662. Moreover, UC mice treated with paeonol showed increased PPARγ expression, which reduced inflammation and apoptosis to maintain intestinal epithelial barrier integrity. In conclusion, our findings suggest that paeonol inhibits the NF-κB signaling pathway by activating PPARγ, reducing inflammation and oxidative stress and improving Dss-induced colitis. This study provides a new insight into the mechanism of treating UC by paeonol.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

