TKI type switching overcomes ROS1 L2086F in ROS1 fusion-positive cancers
- PMID: 39117775
- PMCID: PMC11310217
- DOI: 10.1038/s41698-024-00663-1
TKI type switching overcomes ROS1 L2086F in ROS1 fusion-positive cancers
Erratum in
-
Author Correction: TKI type switching overcomes ROS1 L2086F in ROS1 fusion-positive cancers.NPJ Precis Oncol. 2024 Aug 28;8(1):184. doi: 10.1038/s41698-024-00676-w. NPJ Precis Oncol. 2024. PMID: 39198549 Free PMC article. No abstract available.
Abstract
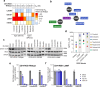
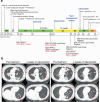
The grammar in this abstract is generally correct, but there's a minor issue with sentence structure in one part. Here's a slightly revised version with improved grammar and flow:ROS1 tyrosine kinase inhibitors (TKIs) are highly effective in ROS1-positive non-small cell lung cancer, but resistance remains a challenge. We investigated the activity of various TKIs against wildtype and mutant ROS1, focusing on the emerging L2086F resistance mutation. Using Ba/F3 and NIH3T3 cell models, CRISPR/Cas9-edited isogenic wildtype and mutant patient-derived cell lines, and in vivo tumor growth studies, we compared type I TKIs (crizotinib, entrectinib, taletrectinib, lorlatinib, and repotrectinib) to type II TKIs (cabozantinib and merestinib) and the type I FLT3 inhibitor gilteritinib. The ROS1 L2086F mutant kinase showed resistance to type I TKIs, while type II TKIs retained activity. Gilteritinib inhibited both wildtype and L2086F mutant ROS1 but was ineffective against the G2032R mutation. Structural analyses revealed distinct binding poses for cabozantinib and gilteritinib, explaining their efficacy against L2086F. Clinical cases demonstrated cabozantinib's effectiveness in patients with TKI-resistant, ROS1 L2086F mutant NSCLCs. This study provides the first comprehensive report of ROS1 L2086F in the context of later-generation TKIs, including macrocyclic inhibitors. While cabozantinib effectively inhibits ROS1 L2086F, its multi-kinase inhibitor nature highlights the need for more selective and better-tolerated TKIs to overcome kinase-intrinsic resistance. Gilteritinib may offer an alternative for targeting ROS1 L2086F with distinct off-target toxicities, but further studies are required to fully evaluate its potential in this setting.
© 2024. The Author(s).
Conflict of interest statement
Dr. Drilon reports Honoraria/Advisory Boards: 14ner/Elevation Oncology, Amgen, Abbvie, ArcherDX, AstraZeneca, Beigene, BergenBio, Blueprint Medicines, Chugai Pharmaceutical, EcoR1, EMD Serono, Entos, Exelixis, Helsinn, Hengrui Therapeutics, Ignyta/Genentech/Roche, Janssen, Loxo/Bayer/Lilly, Merus, Monopteros, MonteRosa, Novartis, Nuvalent, Pfizer, Prelude, Repare RX, Takeda/Ariad/Millenium, Treeline Bio, TP Therapeutics, Tyra Biosciences, Verastem; Associated Research to Institution: Foundation Medicine, Teva, Taiho, GlaxSmithKlein; Equity: mBrace, Treeline; Copyright: Selpercatinib-Osimertinib (pending); Royalties: Wolters Kluwer, UpToDate, Food/Beverage: Boehringer Ingelheim, Merck, Puma: CME Honoraria: Answers in CME, Applied Pharmaceutical Science, Inc., AXIS, Clinical Care Options, EPG Health, Harborside Nexus, I3 Health, Imedex, Liberum, Medendi, Medscape, Med Learning, MJH Life Sciences, MORE Health, Ology, OncLive, Paradigm, Peerview Institute, PeerVoice, Physicians Education Resources, Remedica Ltd., Research to Practice, RV More, Targeted Oncology, TouchIME, WebMD. Dr. Davare reports consulting for Nuvalent. The remaining authors declare no competing interests.
Figures


Update of
-
TKI Type Switching Overcomes ROS1 L2086F in ROS1 Fusion-Positive Cancers.bioRxiv [Preprint]. 2024 Jan 19:2024.01.16.575901. doi: 10.1101/2024.01.16.575901. bioRxiv. 2024. Update in: NPJ Precis Oncol. 2024 Aug 8;8(1):175. doi: 10.1038/s41698-024-00663-1. PMID: 38293020 Free PMC article. Updated. Preprint.
References
Grants and funding
- R01CA273224/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01 CA273224/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- R01 CA233495/CA/NCI NIH HHS/United States
- P30CA008748/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
LinkOut - more resources
Full Text Sources
Miscellaneous

