Effect of immunogenetics polymorphism and expression on direct-acting antiviral drug response in chronic hepatitis C
- PMID: 39117877
- PMCID: PMC11310263
- DOI: 10.1007/s10238-024-01432-x
Effect of immunogenetics polymorphism and expression on direct-acting antiviral drug response in chronic hepatitis C
Abstract
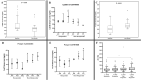
The prevalence of HCV infection in Egypt has decreased following the introduction of direct-acting antiviral therapy. However, treatment response is influenced by various factors, particularly host immunogenetics such as IL-28B and FOXP3 polymorphisms. The current study examined the impact of SNPs in the FOXP3 gene promoter region on HCV-infected Egyptian patients, along with SNPs in the IL28B gene.This study involved 99 HCV patients who achieved SVR12 after a 12 week DAA treatment while 63 HCV patients experienced treatment failure. IL28B rs12979860 SNP was identified using real-time PCR, while IL28B rs8099917, FOXP3 rs3761548, and rs2232365 SNPs were analyzed using RFLP-PCR. Serum levels of IL28B and FOXP3 were quantified using ELISA technique in representative samples from both groups. The IL28B rs12979860 T > C (P = 0.013) and FOXP3 rs2232365 A > G polymorphisms (P = 0.008) were found to significantly increase the risk of non-response. Responders had higher IL28B serum levels (P = 0.046) and lower FOXP3 levels (P < 0.001) compared to non-responders. Regression analysis showed an association between IL28B rs12979860 and FOXP3 rs2232365 with treatment response, independent of age and gender. A predictive model was developed with 76.2% sensitivity and 91.9% specificity for estimating DAAs response in HCV patients.Our findings confirmed the IL28B rs12979860 T > C and FOXP3 rs2232365 A > G polymorphisms significantly affect DAA treatment response in HCV Egyptian patients. Lower levels of IL-28B along with higher levels of FOXP3 are linked to poor response. Our results may lead to new insights into DAA responsiveness contributing to personalized medicine and improving therapeutic decision-making for HCV patients.
Keywords: DAA; Daclatasvir; FOXP3; HCV; IL28B; Sofosbuvir.
© 2024. The Author(s).
Conflict of interest statement
The authors have read the journal’s policy on disclosure of potential conflicts of interest, and they all wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
All authors have read the journal’s authorship statement and agree to it. By your journal’s policy, we confirm that the material contained in the manuscript is original and has not been published and is not being submitted elsewhere. The authors qualify for authorship and have no financial or personal relationships that might lead to a conflict of interest. This manuscript is being submitted online in accordance with your policies for this type of submission.
The Authors hereby grant the Journal full and exclusive rights to the manuscript, all revisions, and the full copyright. The Journal rights include but are not limited to the following: (1) to reproduce, publish, sell, and distribute copies of the manuscript, selections of the manuscript, and translations and other derivative works based upon the manuscript, in print, audio-visual, electronic, or by any media now or hereafter known or devised; (2) to license reprints of the manuscript to third persons for educational photocopying; (3) to license others to create abstracts of the manuscript and to index the manuscript; (4) to license secondary publishers to reproduce the manuscript in print, microform, or any computer-readable form, including electronic on-line databases; and (5) to license the manuscript for document delivery. These exclusive rights run the full term of the copyright, and all renewals and extensions thereof.
Figures



References
-
- World Health Organization: WHO [Internet]. 2023 [cited 2023 Aug 28]. Hepatitis C. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c
-
- Frank C, Mohamed MK, Strickland GT, Lavanchy D, Arthur RR, Magder LS, et al. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000;355(9207):887–91. - PubMed
-
- Esmat G, El-Sayed MH, Hassany M, Doss W, Waked I. One step closer to elimination of hepatitis C in Egypt. Vol. 3, The Lancet Gastroenterology and Hepatology. Elsevier Ltd; 2018. p. 665. - PubMed
-
- Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10(9):553–62. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

